Memory

What is memory?
Memory is the process of encoding, storing, and retrieving experiences and knowledge, and its many guises are even more important than you think.
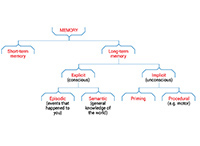
It is hard to overstate the importance of memory. It is what makes us who we are. Some memories are the ones we are aware of – the coffee you enjoyed with a friend, that time as a child when the neighbour’s dog scared you, knowing that spiders have eight legs, and the indisputable fact that the brain is amazing! These are known as explicit memories – ones we can consciously recall.
But there are also implicit memories, which may be even more important. For example, when you talk, you’re using motor memories to move your lips and tongue in a way that reproduces sounds you’ve learnt. When you walk, you’re using motor memories to coordinate your gait.
If we didn’t have memories we’d just be a body, unable to communicate or identify danger and – much like a newborn baby – oblivious to how to survive in the world around us.
In short, memory is crucial in transforming us from helpless newborns into capable adults.