Researchers from UQ’s Queensland Brain Institute (QBI) have revealed the pivotal role played by Synapsin 2a proteins in orchestrating the organisation and mobility of synaptic vesicles within live neurons.
Ms Shanley Longfield, a PhD student in the Meunier lab, explained she used super resolution microscopy, simultaneously tracking key proteins and synaptic vesicles, to make this discovery.
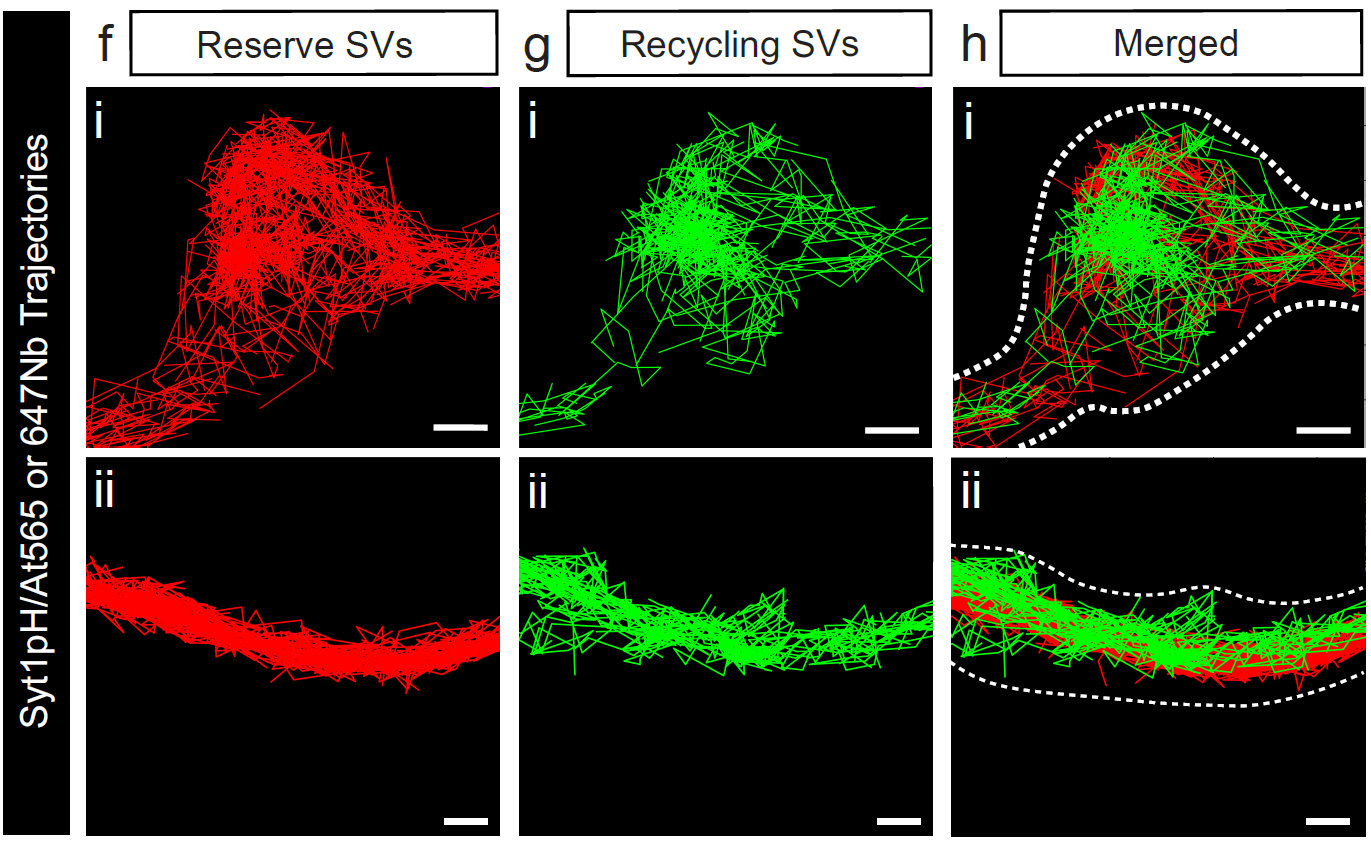
“We used a novel technique, known as dual-pulse sub-diffractional tracking of internalised molecules, developed in our lab, to peer into the inner workings of live hippocampal neurons,” Ms Longfield said.
“One of our main findings revolves around the role of Synapsin 2a tetramerization in controlling the clustering and mobility of reserve synaptic vesicles.
“The reserve synaptic vesicles, which constitute a crucial backup reservoir of neurotransmitters, display lower mobility and are less responsive to high-frequency stimulation compared to their recycling pool counterparts.
“This revelation underscores the intricate balance maintained within neurons to ensure efficient neurotransmitter release under varying physiological conditions.
“Our study also sheds light on the distinct mobility patterns of reserve and recycling synaptic vesicles at different neuronal terminals, emphasising the nuanced regulation of these dynamics by Synapsin proteins.
“Synapsins serve as molecular tethers, anchoring the synaptic vesicles within clusters and fine-tuning their movements, ultimately influencing neurotransmitter release and neuronal communication.
“In earlier research, we have shown that tau, a protein involved in Alzheimer’s disease, controls the dynamic clustering of the important subpopulation of recycling vesicles at the presynapse critical for neuronal communication.
“We found that tau forms small and transient nanoscale biomolecular condensates that can selectively capture these vesicles at the presynapse.
“Now we have tracked the protein – Synapsin 2a – at the same time as the reserve vesicles and shown Synapsin 2a can form tetramers that cross-link synaptic vesicles at the presynapse.”
Professor Fred Meunier said the next step is to study how the two mechanisms, one involving tau and the other Synapsin 2a, “talk to each other” to drive neuronal communication.
“Understanding how condensates and the cross-linking mechanisms coordinate the clustering of synaptic vesicles to allow the presynapse to communicate will be a challenging but exciting endeavour,” Professor Meunier said.
“The implications of this research extend far beyond the realm of basic neuroscience.
“Understanding the precise mechanisms governing synaptic vesicle dynamics deepens our knowledge of fundamental brain processes and holds potential therapeutic applications for neurological disorders, such as Alzheimer’s disease, characterised by disruptions in synaptic function.”
This study was a collaboration with the Ecole Normale Superieure (France), the University of Cambridge (UK), and the Temasek Lifesciences Laboratory (Singapore).
The latest study was published in Nature Communications.



