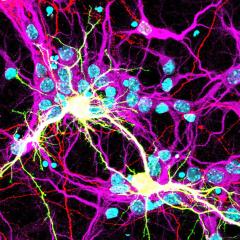
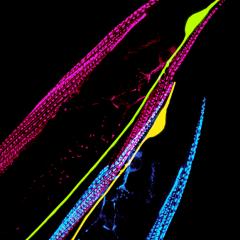
The Hilliard lab is interested in recruiting talented PhD students and Postdocs to join our team. If you're interested in our research, please reach out to Massimo Hilliard at m.hilliard@uq.edu.au.
The Hilliard laboratory is focused on understanding the molecular mechanisms that regulate neuronal development, maintenance and repair, using C. elegans as a model system. The group’s current research goals are: (1) how the axon, which is the longest of the neuronal processes, is subdivided into structurally and functionally different compartments, (2) how the axon maintains its structure and function over the lifetime of the organism, and (3) how the axon can be repaired when severing damage occurs.
Using a combination of molecular biology, genetics, laser manipulations, and imaging approaches, the Hilliard group has made a number of key discoveries in these research areas. They include: the axonal protective function of a conserved alpha tubulin acetyltransferase (Cell Reports, 2014); the role of conserved apoptotic molecules in axonal degeneration; and the identification of the molecular mechanisms that regulate axonal fusion, an axonal repair event in which the two separated fragments of an injured axon rejoin and reconstitute the original tract (Nature, 2015). This latest discovery on how axonal fusion is achieved has important implications for medical practice: a similar strategy may be used to facilitate nerve repair.
The Hilliard group has been successful in attracting competitive funding that includes an ARC Discovery Project, an NHMRC Senior Research Fellowship, and a NHMRC-ARC Dementia Fellowship.
Podcast
Group leader

Professor Massimo Hilliard
NHMRC Leadership Fellow - GL, Queensland Brain Institute
+61 7 334 66390
@HilliardLab
m.hilliard@uq.edu.au
UQ Researcher Profile

Axonal degeneration
How neurons can maintain their axonal structure and function over time is not well understood. Axonal degeneration is a critical and common feature of many peripheral neuropathies, neurodegenerative diseases and nerve injuries. The genetic factors and the cellular mechanisms that prevent axonal degeneration under normal conditions and that trigger it under pathological ones are still largely unknown. We aim to use C. elegans genetics to identify the molecules and the mechanisms that control these processes.
Axonal regeneration
How some axons can regenerate after nerve damage while others cannot is a crucial question in neurobiology, and the answers will be of great value for the medical handling of neurodegenerative diseases and of traumatic nerve injuries. Largely unknown are the molecules and the mechanisms underlying this important biological process. In C. elegans, a new laser-based technology allows single neuron axotomy in living animals, and axonal regeneration can now be visualised in real-time and tackled with a genetic approach. Our goal is to identify the genes and conditions that control this fascinating process.
Neuronal polarity and axonal guidance
Neurons are highly polarized cells with distinct domains such as axons and dendrites. The polarity of a developing neuron determines the precise exit point of its axon as well as the initial trajectory of axon outgrowth. Understanding how neurons establish and orient polarity with respect to extracellular cues is an important and challenging problem in neurobiology. We wish to understand how different secreted cues regulate the orientation of neuronal polarity and axonal/dendrite guidance in vivo.
Donato, A., Ritchie, F. K., Lu, L., Wadia, M., Martinez-Marmol, R., Kaulich, E., Sankorrakul, K., Lu, H., Coakley, S., Coulson, E. J., Hilliard, M. A. OSP-1 protects neurons from autophagic cell death induced by acute oxidative stress. Nature Communications, 2025, 16: 300. doi: 10.1038/s41467-024-55105-0 (Featured in Editors’ highlights)
Martínez-Mármol, Ramón, Giordano-Santini, Rosina, Kaulich, Eva, Cho, Ann-Na, Przybyla, Magdalena, Riyadh, Md Asrafuzzaman, Robinson, Emilija, Chew, Keng Yih, Amor, Rumelo, Meunier, Frédéric A., Balistreri, Giuseppe, Short, Kirsty R., Ke, Yazi D., Ittner, Lars M. and Hilliard, Massimo A. (2023). SARS-CoV-2 infection and viral fusogens cause neuronal and glial fusion that compromises neuronal activity. Science Advances, 9 (23) eadg2248, 1-15. doi: 10.1126/sciadv.adg2248
Vijayaraghavan, Tarika, Dhananjay, Samiksha, Ho, Xue Yan, Giordano-Santini, Rosina, Hilliard, Massimo and Neumann, Brent (2023). The dynamin GTPase mediates regenerative axonal fusion in Caenorhabditis elegans by regulating fusogen levels. PNAS Nexus, 2 (5). doi: 10.1093/pnasnexus/pgad114
Ding, Qiao, Kesavan, Kaamini, Lee, Kah Meng, Wimberger, Elyse, Robertson, Thomas, Gill, Melinder, Power, Dominique, Chang, Jeryn, Fard, Atefeh T., Mar, Jessica C., Henderson, Robert D., Heggie, Susan, McCombe, Pamela A., Jeffree, Rosalind L., Colditz, Michael J., Hilliard, Massimo A., Ng, Dominic C. H., Steyn, Frederik J., Phillips, William D., Wolvetang, Ernst J., Ngo, Shyuan T. and Noakes, Peter G. (2022). Impaired signaling for neuromuscular synaptic maintenance is a feature of Motor Neuron Disease. Acta Neuropathologica Communications, 10 (1) 61, 61. doi: 10.1186/s40478-022-01360-5
Ho, X. Y., Coakley, S., Amor, R., Anggono, V., and Hilliard, M.A. The metalloprotease ADM-4/ADAM17 promotes axonal repair. Science Advances, 2022, 8: eabm2882. (Online featured).
Bonacossa-Pereira, I., Coakley, S., and Hilliard, M.A. Neuron-epidermal attachment protect hyper-fragile axons from mechanical strain. Cell Reports, 2022, 38: 1100501. (Cover image).
Ding, Q., Chaplin, J., Morris, M.J., Hilliard, M.A., Wolvetang, E., Ng, D.C.H., and Noakes, P. TDP-43 mutation affects stress granule dynamics in differentiated NSC-34 motoneuron-like cells. Frontiers in Cell and Developmental Biology, 2021, 9: doi: 10.3389/fcell.2021.611601.
Gormal, R.S., Padmanabhan, P., Kasula, R., Bademosi, A.T., Coakley, S., Giacomotto, J., Blum, A., Joensuu, M., Wallis, T.P., Lo, H.P., Budnar, S., Rae, J., Ferguson, C., Bastiani, M., Thomas, W.G., Pardon, E., Steyaert, J., Yap, A.S., Goodhill, G.J., Hilliard, M.A., Parton, R.G., and Meunier, F.A. Modular transient nanoclustering of activated b2-adrenergic receptors revealed by single-molecule tracking of conformation-specific nanobodies. PNAS, 2020, 117: 30476-30487.
Giordano-Santini, R., Kaulich, E., Galbraith, K.M., Ritchie, F.K., Wang, W., Li, Z., and Hilliard, M.A. Fusogen-mediated neuron-neuron fusion disrupts neural circuit connectivity and alters animal behavior. PNAS, 2020, 117: 23054-23065.
Coakley, S., Ritchie, F., Galbraith, K., and Hilliard M.A. Epidermal control of axonal attachment via ß-spectrin and the GTPase-activating protein TBC-10 prevents axonal degeneration. Nature Communications, 2020, 11: 133. doi: 10.1038/s41467-019-13795-x.
Neumann, B., and Hilliard, M.A. Axonal repair by fusion: pitfalls, consequences and solutions. FASEB J., 2019, 33: 13071-13074.
Linton, C., Riyadh, M.A., Ho, X.H., Neumann, B., Giordano-Santini, R.* and Hilliard, M.A.* Disruption of RAB-5 increases EFF-1 fusogen availability at the cell surface and promotes the regenerative axonal fusion capacity of the neuron. Journal of Neuroscience, 2019, 39: 2823-2836. (Cover image).
Neumann, B.*, Linton, C, Giordano-Santini, R., and Hilliard M.A.* Axonal fusion: an alternative and efficient mechanism of axonal repair. Progress in Neurobiology, 2019, 173: 88-101.
Donato, A.≠, Kagias, K.≠, Zhang, Y.* and Hilliard M.A.* Neuronal sub-compartmentalization: a strategy to optimize neuronal function. Biological Reviews, 2019, 94: 1023-1037. doi: 10.1111/brv.12487.
Offenburger, S.L., Ho, X.Y., Tachie-Menson T., Coakley, S., Hilliard, M.A. and Gartner, A. 6-OHDA-induced dopaminergic neurodegeneration in Caenorhabditis elegans is promoted by the engulfment pathway and inhibited by the transthyretin-related protein TTR-33. PLoS Genetics, 2018, 14: e1007125. doi: 10.1371/journal.pgen.1007125.
Chand K.K., Lee, K.M., Lee, J.D., Hao, Q., Willis, E.F., Lavidis, N.A., Hilliard, M.A. and Noakes, P.G. Defects in synaptic transmission at the neuromuscular junction precedes motor deficits in TDP-43Q331K transgenic mouse model of amyotrophic lateral sclerosis. FASEB J., 2018, doi: 10.1096/fj.201700835R.
Abay, Z., Wong, Y., M., Teoh, J.S. Vijayaraghavan, T, Hilliard, M.A. and Neumann, B. Phosphatidylserine ‘save-me’ signals drive functional recovery of severed axons in Caenorhabditis elegans. PNAS, 2017, 114: E10196-10205.
Ritchie F.K.*, Knable, R.*, Chaplin, J.*, Gurnsansky, R., Gallegos M., Neumann, B., and Hilliard, M.A. The heterochronic gene lin-14 controls axonal degeneration in C. elegans neurons. Cell Reports, 2017, 20: 2955-2965.
Gorke, S.K., Hegarty, E.M., Mondal, S., Zhao, P., Ghorashian, N., Hilliard, M.A., and Ben-Yakar A. A multi-trap microfluidic chip enabling longitudinal studies of nerve regeneration in Caenorhabditis elegans. Scientific Reports, 2017, 7: 9837. doi:10.1038/s41598-017-10302-4.
Fogarty, M.J., Klenowski, P.M., Lee, J.D., Drieberg-Thompson, J.R., Bartlett, S.E., Ngo, S.T., Hilliard, M.A., Bellingham, M.C., and Noakes, P.G. Cortical synaptic and dendritic spine abnormalities in a presymptomatic TDP-43 model of amyotrophic lateral sclerosis. Scientific Reports, 2016. doi:10.1038/srep37968.
Giordano-Santini, R.*, Linton, C., and Hilliard, M.A.* Cell-cell fusion in the nervous system: Alternative mechanisms of development, injury, and repair. *Corresponding authors. Seminars in Cell & Developmental Biology, 2016, 60: 146-154.
Gallotta, I., Mazzarella, N., Donato, A., Esposito, A., Chaplin, J.C., Silvana Castro, S., Zampi, G., Battaglia, G.S., Hilliard, M.A., Bazzicalupo, P., and Di Schiavi, E. Neuron-specific knock-down of SMN1 causes neuron degeneration and death through an apoptotic mechanism. Human Molecular Genetics, 2016, 25: 2564-2577.
Nichols, A.L.A., Meelkop, E., Linton, C., Giordano-Santini, R., Sullivan, R.K., Donato, A., Nolan C., Hall, D.H, Xue, D., Neumann, B.* and Hilliard, M.A.* The apoptotic engulfment machinery regulates axonal degeneration in C. elegans neurons. Cell Reports, 2016, 14: 1673-1683. *Corresponding authors.
Neumann, B., Coakley, S., Giordano-Santini, R., Linton, C., Lee, E.S., Nakagawa, A., Xue, D. and Hilliard, M.A. EFF-1-mediated regenerative axonal fusion requires components of the apoptotic pathway. Nature, 2015, 517: 219-222. (Cover image).
Lee H., Kim, S.A., Coakley, S., Mugno, P., Hammarlund, M., Hilliard M.A. and Lu, H. A multi-channel device for high-density target-selective stimulation and long-term monitoring of cells and subcellular features in C. elegans. Lab on a Chip, 2014, 14: 4513-4522.
Neumann, B. and Hilliard, M.A. Loss of MEC-17 leads to microtubule instability and axonal degeneration. Cell Reports, 2014, 6: 93-103.
Williams, D.C., El Bejjani, R., Mugno Ramirez, P., Coakley, S., Kim, S.A. Lee, H., Wen, Q., Samuel, A., Lu, H.*, Hilliard, M.A.*, and Hammarlund, M.* Rapid and permanent neuronal inactivation in vivo via subcellular generation of reactive oxygen with the use of KillerRed. Cell Reports, 2013, 5: 553-563. *Corresponding authors.
Kirszenblat, L., Neumann, B., Coakley, S., and Hilliard, M.A. A dominant mutation in MEC-7/β-tubulin affects axon development and regeneration in Caenorhabditis elegans neurons. Molecular Biology of the Cell, 2013, 24: 285-296. (Cover image).
Schlipalius, D.I., Valmas, N., Tuck, A.G., Jagadeesan, R., Ma, L., Kaur, R., Goldinger, A., Anderson, C., Kuang, J., Zuryn, S., Mau, Y.S., Cheng, Q., Collins, P.J., Nayak, M.K., Schirra, H.J., Hilliard, M.A.,* and Ebert, P.R. * A core metabolic enzyme mediates resistance to phosphine gas. Science, 2012, 338: 807-810. *Corresponding authors.
Cáceres, I.dC., Valmas, N., Hilliard, M.A., and Lu, H. Laterally orienting C. elegans using geometry at microscale for high-throughput visual screens in neurodegeneration and neuronal development studies. PLoS One, 2012, 7: e35037. doi:10.1371/journal.pone.0035037. (Selected by the Faculty of 1000).
Kirszenblat, L., Pattabiraman, D., and Hilliard, M.A. LIN-44/Wnt directs dendrite outgrowth through LIN-17/Frizzled in C. elegans neurons. PLoS Biology, 2011, 9: e1001157. doi:10.1371/journal.pbio.1001157. (Synopsis in the same issue).
Neumann, B., Nguyen, K.C.Q., Hall, D.H., Ben-Yakar, A. and Hilliard, M.A. Axonal regeneration proceeds through specific axonal fusion in transected C. elegans neurons. Developmental Dynamics, 2011, 240: 1365-1372. (Cover image, highlighted in the Editor’s choice in Science).
Burne, T., Scott, E., van Swinderen, B., Hilliard, M., Reinhard, J., Claudianos, C., Eyles, D., and McGrath, J. Big ideas for small brains: what can psychiatry learn from worms, flies, bees and fish? Molecular Psychiatry, 2010, 16: 7-16. (Cover image).
Hilliard, M.A. Axonal degeneration and regeneration: a mechanistic tug-of-war. Journal of Neurochemistry, 2009, 108: 23-32.
Guo, S.X., Bourgeois, F., Chokshi, T., Durr, N.J., Hilliard, M.A., Chronis, N. and Ben-Yakar, A. Femtosecond laser nanoaxotomy lab-on-a-chip for in vivo nerve regeneration studies. Nature Methods, 2008, 5: 531-533.
Chalasani, S.H., Feinberg, E.H. and Hilliard, M.A. Global ‘worming’. Genome Biology, 2007, 8: 314. (Report on the 16th International C. elegans Meeting).
Hilliard, M.A. and Bargmann, C.I. Wnt signals and Frizzled activity orient anterior-posterior axon outgrowth in C. elegans. Developmental Cell, 2006, 10: 379-390. (Selected by Faculty of 1000).
Pan, C.L., Howell, J.E., Clark, S.G., Hilliard, M.A., Cordes, S., Bargmann, C.I. and Garriga, G. Multiple Wnt homologs regulate anteriorly directed cell and growth cone migrations in Caenorhabditis elegans. Developmental Cell, 2006, 10: 367-377. (Selected by Faculty of 1000).
Sapio, M.R.*, Hilliard, M.A.*, Cermola, M., Favre, R. and Bazzicalupo, P. The Zona Pellucida domain containing proteins, CUT-1, CUT-3 and CUT-5, play essential roles in the development of the larval alae in Caenorhabditis elegans. Developmental Biology, 2005, 282: 231-245. * Equal contribution.
Hilliard, M.A.*, Apicella, A.J.*, Kerr, R.*, Suzuki, H., Bazzicalupo, P. and Schafer, W.R. In vivo imaging of C. elegans ASH neurons: cellular response and adaptation to chemical repellents. EMBO Journal, 2005, 24: 63-72. * Equal contribution.
Hilliard, M.A., Bergamasco, C., Arbucci, S., Plasterk, R.H.A. and Bazzicalupo, P. Worms taste bitter: ASH neurons, QUI-1, GPA-3 and ODR-3 mediate quinine avoidance in Caenorhabditis elegans. EMBO Journal, 2004, 23: 1101-1111.
Hilliard, M.A., Bargmann, C.I. and Bazzicalupo, P. C. elegans responds to chemical repellents by integrating sensory inputs from the head and the tail. Current Biology, 2002, 12: 730-734. (Dispatch in the same issue).
Rinaldo, C., Bazzicalupo, P., Ederle, S., Hilliard, M.A. and La Volpe, A. Roles for Caenorhabditis elegans rad-51 in meiosis and in resistance to ionizing radiation during development. Genetics, 2002, 160: 471-479.
Rugarli, E.I., Di Schiavi, E., Hilliard, M.A., Arbucci, S., Ghezzi, C., Facciolli, A., Coppola, G., Ballabio, A. and Bazzicalupo, P. The Kallmann syndrome gene homolog in C. elegans is involved in epidermal morphogenesis and neurite branching. Development, 2002, 129: 1283 1294. (Selected by Faculty of 1000).
Salcini, A.E., Hilliard, M.A., Croce, A., Arbucci, S., Luzzi, P., Tacchetti, C., Daniell, L., De Camilli, P., Pelicci, P.G., Di Fiore, P.P. and Bazzicalupo, P. The Eps15 C. elegans homologue EHS-1 is implicated in synaptic vesicle recycling. Nature Cell Biology, 2001, 3: 755-760. (Selected by Faculty of 1000).
Bazzicalupo, P., Hilliard, M.A., Lewis, E., De Riso, L., Sebastiano, M. and Ristoratore, F. Neurons and genes involved in chemical sensitivity in nematodes. Parasite, 1994, 1: 58-60.
- Associate Professor Hang Lu - Georgia Institute of Technology, Atlanta, USA
- Associate Professor Yun Zhang - Harvard University, Cambridge, USA
- Professor Ding Xue - University of Colorado, Boulder, USA.
- Prof. Fred Meunier - QBI, The University of Queensland, Brisbane, Australia
- A/Prof Peter Noakes - SMBS, The University of Queensland, Brisbane, Australia
- Dr Paolo Bazzicalupo and Dr Elia Di Schiavi - Institute of Genetics and Biophysics, Naples, Italy
- Professor Paul Ebert - The University of Queensland, Brisbane, Australia
Research Areas
- Axonal degeneration
- Axonal regeneration
- Neuronal development
Latest news
-
-
COVID-19 can cause brain cells to ‘fuse’
8 June 2023 -
What regulates the 'glue’ needed for nerve repair?
17 March 2022