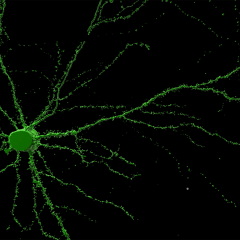
The overall goal of our research is to determine how brain cells communicate and survive in health and disease. Our lab focuses on the molecular events that govern vesicular trafficking within presynaptic nerve terminals and neurosecretory cells. Our discoveries have led to a deep understanding of how secretory vesicles interact with the cortical actin network prior to fusing with the plasma membrane to release the neurotransmitter. We have identified a critical role of the phospholipid phosphatidylinositol (4,5) bisphosphate in coordinating the actin-mediated recruitment of secretory vesicles to the plasma membrane. This active mechanism allows secretory vesicles to dock with the plasma membrane prior to fusion and is controlled by the effector cdc42. We have recently uncovered a new mechanism that allows secretory vesicles to be recruited on the cortical actin network.
We demonstrated that the protein Munc18-1 was responsible for the transport of syntaxin-1 to the plasma membrane. This novel trafficking/chaperoning pathway underpins neurotransmission, as secretory vesicles rely on the zippering of SNARE proteins such as syntaxin-1 to undergo fusion with the plasma membrane. Following exocytosis, the process of endocytosis is required for neurons to replenish their pools of synaptic vesicles by pinching off vesicles from the plasma membrane. We have identified a novel mechanism by which dynamin and actin coordinate a series of membrane events that culminate in the formation of bulk endosomes from which new synaptic vesicles emanate at the neuromuscular junction. We have also demonstrated that dynamin is a valid prophylactic target against infection and intoxication.
As part of the Clem Jones Centre for Ageing Dementia Research, the Meunier laboratory has capitalised on the recent acquisition of several super-resolution microscopes to uncover how neurotrophic factors are packaged in nanodomains of presynaptic terminals and then channelled back to the cell body.
Our lab is keen on taking on highly motivated and high achieving students (Honours and PhD).
Group leader

Professor Frederic Meunier
Professor, Queensland Brain Institute
@MeunierLab
Laboratory Facebook Group
+61 7 334 66373
f.meunier@uq.edu.au
UQ Researcher Profile
Super-resolution microscopy at the site of neuronal communication
QBI houses the Advanced Microimaging and Analysis Facility, which contains state-of-the art microscopes. Our lab has been instrumental in the inclusion of several super-resolution microscopes, through successive ARC LIEF grants. This has allowed us to probe the nanoscopic environment of neurons and neurosecretory cells undergoing communication. Our most recent publications have used super-resolution microscopy extensively.
Deciphering the intra- and intermolecular steps via which prepare secretory vesicles for fusion is key to understanding neuronal and hormonal communication. We demonstrated that Munc18-1 and syntaxin-1A and are organised in nanodomains on the plasma membrane of neurons and neurosecretory cells that control SNARE-dependent neuroexocytosis through lateral trapping in these nanoclusters. In Bademosi et al. 2017 (Nature Communications), we combined super-resolution with opto- and thermogenetic neuronal stimulation in living neurons in the fruit fly. We were able to characterise the changes in mobility of the tSNARE syntaxin1a and how they regulate neurotransmitter release. In Kasula et al. 2016 (Journal of Cell Biology) we found that the Munc18-1 domain 3a hinge-loop controls syntaxin-1A engagement into the SNARE complex during priming.
We also developed a new technique sdTIM, Subdiffractional tracking of internalised molecules, to be able to visualise small synaptic vesicles in living hippocampal nerve terminals (Joensuu et al. 2016 Journal of Cell Biology). This super-resolution technique was able to capture, and subsequently analyse, the dynamics of thousands of individual synaptic vesicle trajectories, to uncover the dynamics of synaptic vesicle pool mobility. It revealed diffusive and transport states of synaptic vesicles in resting and stimulated conditions. Our aim is to apply sdTIM to study the mechanism of neuronal communication under disease conditions in future studies.
Munc18 and synucleopathies
Because of the importance of Munc18-1 in vesicle fusion, human mutations are linked to epilepsy and other neurological symptoms. Recent genetic studies in humans have uncovered a variety of mutations in Munc18 that lead to EIEE (early infantile epileptic encephalopathy), a severe and often fatal developmental condition with no treatment, and little knowledge of the underlying cause. We showed that the human disease- linked mutation C180Y, which is unstable at body temperature, leads to the formation of intracellular aggregates (Martin et al. 2014 Cell Reports). In a follow up study, we revealed that both C180Y and other human EIEE-linked missense mutations controlled the aggregation of α-synuclein, in a similar fashion to Parkinson's disease pathology (Chai et al. 2016 Journal of Cell Biology). This provides the first evidence for a communal mode of action between an epileptic syndrome and neurodegenerative synucleopathies. This is an ongoing field of research in our laboratory.
Selected journal publications
Saturated free fatty acids and association with memory formation
Wallis, Tristan P., Venkatesh, Bharat G., Narayana, Vinod K., Kvaskoff, David, Ho, Alan, Sullivan, Robert K., Windels, François, Sah, Pankaj and Meunier, Frédéric A. (2021). Saturated free fatty acids and association with memory formation. Nature Communications, 12 (1) 3443, 3443. doi: 10.1038/s41467-021-23840-3
Modular transient nanoclustering of activated β2-adrenergic receptors revealed by single-molecule tracking of conformation-specific nanobodies
Gormal, Rachel S., Padmanabhan, Pranesh, Kasula, Ravikiran, Bademosi, Adekunle T., Coakley, Sean, Giacomotto, Jean, Blum, Ailisa, Joensuu, Merja, Wallis, Tristan P., Lo, Harriet P., Budnar, Srikanth, Rae, James, Ferguson, Charles, Bastiani, Michele, Thomas, Walter G., Pardon, Els, Steyaert, Jan, Yap, Alpha S., Goodhill, Geoffrey J., Hilliard, Massimo A., Parton, Robert G. and Meunier, Frédéric A. (2020). Modular transient nanoclustering of activated β2-adrenergic receptors revealed by single-molecule tracking of conformation-specific nanobodies. Proceedings of the National Academy of Sciences of the United States of America, 117 (48), 30476-30487. doi: 10.1073/pnas.2007443117
Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity
Cantuti-Castelvetri, Ludovico, Ojha, Ravi, Pedro, Liliana D., Djannatian, Minou, Franz, Jonas, Kuivanen, Suvi, van der Meer, Franziska, Kallio, Katri, Kaya, Tuğberk, Anastasina, Maria, Smura, Teemu, Levanov, Lev, Szirovicza, Leonora, Tobi, Allan, Kallio-Kokko, Hannimari, Österlund, Pamela, Joensuu, Merja, Meunier, Frédéric A., Butcher, Sarah J., Winkler, Martin Sebastian, Mollenhauer, Brit, Helenius, Ari, Gokce, Ozgun, Teesalu, Tambet, Hepojoki, Jussi, Vapalahti, Olli, Stadelmann, Christine, Balistreri, Giuseppe and Simons, Mikael (2020). Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science, 370 (6518) abd2985, 856-860. doi: 10.1126/science.abd2985
Radial contractility of actomyosin rings facilitates axonal trafficking and structural stability
Wang, Tong, Li, Wei, Martin, Sally, Papadopulos, Andreas, Joensuu, Merja, Liu, Chunxia, Jiang, Anmin, Shamsollahi, Golnoosh, Amor, Rumelo, Lanoue, Vanessa, Padmanabhan, Pranesh and Meunier, Frédéric A. (2020). Radial contractility of actomyosin rings facilitates axonal trafficking and structural stability. Journal of Cell Biology, 219 (5) e201902001, 1-S7. doi: 10.1083/jcb.201902001
p110δ PI 3-kinase inhibition perturbs APP and TNFα trafficking, reduces plaque burden, dampens neuroinflammation and prevents cognitive decline in an Alzheimer's disease mouse model
Martínez-Mármol, Ramón, Mohannak, Nika, Qian, Lei, Wang, Tong, Gormal, Rachel S, Ruitenberg, Marc J, Vanhaesebroeck, Bart, Coulson, Elizabeth J and Meunier, Frédéric A (2019). p110δ PI 3-kinase inhibition perturbs APP and TNFα trafficking, reduces plaque burden, dampens neuroinflammation and prevents cognitive decline in an Alzheimer's disease mouse model. Journal of Neuroscience, 39 (40), 7976-7991. doi: 10.1523/JNEUROSCI.0674-19.2019
STXBP1 encephalopathy: connecting neurodevelopmental disorders with α-synucleinopathies?
Lanoue, Vanessa, Chai, Ye Jin, Brouillet, Julie Z., Weckhuysen, Sarah, Palmer, Elizabeth E., Collins, Brett M. and Meunier, Frederic A. (2019). STXBP1 encephalopathy: connecting neurodevelopmental disorders with α-synucleinopathies?. Neurology, 93 (3), 114-123. doi: 10.1212/WNL.0000000000007786
Frontotemporal dementia mutant Tau promotes aberrant Fyn nanoclustering in hippocampal dendritic spines
Padmanabhan, Pranesh, Martínez-Mármol, Ramón, Xia, Di, Götz, Jürgen and Meunier, Frédéric A. (2019). Frontotemporal dementia mutant Tau promotes aberrant Fyn nanoclustering in hippocampal dendritic spines. eLife, 8 e45040. doi: 10.7554/elife.45040
Trapping of Syntaxin1a in Presynaptic Nanoclusters by a Clinically Relevant General Anesthetic.
Bademosi, Adekunle T. , Steeves, James, Karunanithi, Shanker, Zalucki, Oressia H, Gormal, Rachel S, Liu, Shu, Lauwers, Elsa, Verstreken, Patrik, Anggono, Victor, Meunier, Frederic A and van Swinderen, Bruno (2018). Cell reports, 22 2: 427-440. doi:10.1016/j.celrep.2017.12.054
Amyloid-β and tau complexity - towards improved biomarkers and targeted therapies. Polanco, Juan Carlos, Li, Chuanzhou, Bodea, Liviu-Gabriel, Martinez-Marmol, Ramon, Meunier, Frederic A and Götz, Jürgen (2017). Nature reviews. Neurology, 14 1: 22-40. doi:10.1038/nrneurol.2017.162
Visualizing endocytic recycling and trafficking in live neurons by subdiffractional tracking of internalized molecules.
Joensuu, Merja, Martinez-Marmol, Ramon, Padmanabhan, Pranesh, Glass, Nick R., Durisic, Nela, Pelekanos, Matthew, Mollazade, Mahdie, Balistreri, Giuseppe, Amor, Rumelo, Cooper-White, Justin J., Goodhill, Geoffrey J. and Meunier, Frederic A. (2017) Nature Protocols, 12 12:2590-2622. doi:10.1038/nprot.2017.116
The SAC1 domain in synaptojanin is required for autophagosome maturation at presynaptic terminals.
Vanhauwaert, Roeland, Kuenen, Sabine, Masius, Roy, Bademosi, Adekunle, Manetsberger, Julia, Schoovaerts, Nils, Bounti, Laura, Gontcharenko, Serguei, Swerts, Jef, Vilain, Sven, Picillo, Marina, Barone, Paolo, Munshi, Shashini T., de Vrij, Femke M. S., Kushner, Steven A., Gounko, Natalia V., Mandemakers, Wim, Bonifati, Vincenzo, Meunier, Frederic A., Soukup, Sandra‐Fausia and Verstreken, Patrik (2017)EMBO Journal, 36 10: 1392-1411. doi:10.15252/embj.201695773
In vivo single-molecule imaging of syntaxin1A reveals polyphosphoinositide- and activity-dependent trapping in presynaptic nanoclusters
Bademosi, Adekunle T., Lauwers, Elsa, Padmanabhan, Pranesh, Odierna, Lorenzo, Chai, Ye Jin, Papadopulos, Andreas, Goodhill, Geoffrey J., Verstreken, Patrik, Van Swinderen, Bruno and Meunier, Frederic A. (2017) Nature Communications, 8 . doi:10.1038/ncomms13660
Subdiffractional tracking of internalized molecules reveals heterogeneous motion states of synaptic vesicles
Joensuu, Merja, Padmanabhan, Pranesh, Durisic, Nela, Bademosi, Adekunle T. D., Cooper-Williams, Elizabeth, Morrow, Isabel C., Harper, Callista B., Jung, WooRam, Parton, Robert G., Goodhill, Geoffrey J., Papadopulos, Andreas and Meunier, Frederic A. (2016) Journal of Cell Biology, 215 2: 277-292. doi:10.1083/jcb.201604001
Flux of signalling endosomes undergoing axonal retrograde transport is encoded by presynaptic activity and TrkB
Wang, Tong, Martin, Sally, Nguyen, Tam H., Harper, Callista B., Gormal, Rachel S., Martinez-Marmol, Ramon, Karunanithi, Shanker, Coulson, Elizabeth J., Glass, Nick R., Cooper-White, Justin J., Van Swinderen, Bruno and Meunier, Frederic A. (2016) Nature Communications, 7 . doi:10.1038/ncomms12976
The Munc18-1 domain 3a hinge-loop controls syntaxin-1A nanodomain assembly and engagement with the SNARE complex during secretory vesicle priming
Kasula, Ravikiran, Chai, Ye Jin, Bademosi, Adekunle T., Harper, Callista B., Gormal, Rachel S., Morrow, Isabel C., Hosy, Eric, Collins, Brett M., Choquet, Daniel, Papadopulos, Andreas and Meunier, Frederic A. (2016) The Journal of Cell Biology, 214 7: 847-858. doi:10.1083/jcb.201508118
Munc18-1 is a molecular chaperone for α-synuclein, controlling its self-replicating aggregation
Chai, Ye Jin, Sierecki, Emma, Tomatis, Vanesa M., Gormal, Rachel S., Giles, Nichole, Morrow, Isabel C., Xia, Di, Götz, Jürgen, Parton, Robert G., Collins, Brett M., Gambin, Yann and Meunier, Frédéric A. (2016) The Journal of Cell Biology, 214 6: 705-718. doi:10.1083/jcb.201512016
Profiling of free fatty acids using stable isotope tagging uncovers a role for saturated fatty acids in neuroexocytosis
Narayana, Vinod K., Tomatis, Vanesa M., Wang, Tong, Kvaskoff, David and Meunier, Frederic A. (2015) Chemistry and Biology, 22 11: 1552-1561. doi:10.1016/j.chembiol.2015.09.010
Small molecules demonstrate the role of dynamin as a bi-directional regulator of the exocytosis fusion pore and vesicle release
Jackson, J., Papadopulos, A., Meunier, F. A., McCluskey, A., Robinson, P. J. and Keating, D. J. (2015) Molecular Psychiatry, 20 7: 810-819. doi:10.1038/mp.2015.56
Control of autophagosome axonal retrograde flux by presynaptic activity unveiled using botulinum neurotoxin type A
Wang, Tong, Martin, Sally, Papadopulos, Andreas, Harper, Callista B., Mavlyutov, Timur A., Niranjan, Dhevahi, Glass, Nick R., Cooper-White, Justin J., Sibarita, Jean-Baptiste, Choquet, Daniel, Davletov, Bazbek and Meunier, Frederic A. (2015) Journal of Neuroscience, 35 15: 6179-6194. doi:10.1523/JNEUROSCI.3757-14.2015
Activity-driven relaxation of the cortical actomyosin II network synchronizes Munc18-1-dependent neurosecretory vesicle docking
Papadopulos, Andreas, Gomez, Guillermo A., Martin, Sally, Jackson, Jade, Gormal, Rachel S., Keating, Damien J., Yap, Alpha S. and Meunier, Frederic A. (2015) Nature Communications, 6 6297: 1-11. doi:10.1038/ncomms7297
An acto-myosin II constricting ring initiates the fission of activity-dependent bulk endosomes in neurosecretory cells
Gormal, Rachel S, Nguyen, Tam H, Martin, Sally, Papadopulos, Andreas and Meunier, Frederic A (2015) Journal of Neuroscience, 35 4: 1380-1389. doi:10.1523/JNEUROSCI.3228-14.2015
Increased polyubiquitination and proteasomal degradation of a Munc18-1 disease-linked mutant causes temperature-sensitive defect in exocytosis
Martin, Sally, Papadopulos, Andreas, Tomatis, Vanesa M., Sierecki, Emma, Malintan, Nancy T., Gormal, Rachel S., Giles, Nichole, Johnston, Wayne A., Alexandrov, Kirill, Gambin, Yann, Collins, Brett M. and Meunier, Frederic A. (2014) Cell Reports, 9 1: 206-218. doi:10.1016/j.celrep.2014.08.059
PI3K delta inhibition reduces TNF secretion and neuroinflammation in a mouse cerebral stroke model
Low, Pei Ching, Manzanero, Silvia, Mohannak, Nika, Narayana, Vinod K., Nguyen, Tam H., Kvaskoff, David, Brennan, Faith H., Ruitenberg, Marc J., Gelderblom, Mathias, Magnus, Tim, Kim, Hyun Ah, Broughton, Brad R. S., Sobey, Christopher G., Vanhaesebroeck, Bart, Stow, Jennifer L., Arumugam, Thiruma V. and Meunier, Frédéric A. (2014) Nature Communications, 5 3450: 1-12. doi:10.1038/ncomms4450
Myosin VI small insert isoform maintains exocytosis by tethering secretory granules to the cortical actin
Tomatis, Vanesa M., Papadopulos, Andreas, Malintan, Nancy T., Martin, Sally, Wallis, Tristan, Gormal, Rachel S., Kendrick-Jones, John, Buss, Folma and Meunier, Frederic A. (2013) Journal of Cell Biology, 200 3: 301-320. doi:10.1083/jcb.201204092
Secretagogue stimulation of neurosecretory cells elicits filopodial extensions uncovering new functional release sites
Papadopulos, Andreas, Martin, Sally, Tomatis, Vanesa M., Gormal, Rachel S. and Meunier, Frederic A. (2013) Secretagogue stimulation of neurosecretory cells elicits filopodial extensions uncovering new functional release sites.Journal of Neuroscience, 33 49: 19143-19153. doi:10.1523/JNEUROSCI.2634-13.2013
Targeting membrane trafficking in infection prophylaxis: dynamin inhibitors
Harper, Callista B., Popoff, Michel R., McCluskey, Adam, Robinson, Phillip J. and Meunier, Frederic A. (2013) Trends in Cell Biology, 23 2: 90-101. doi:10.1016/j.tcb.2012.10.007
Phosphatidylinositol(4,5)bisphosphate coordinates actin-mediated mobilization and translocation of secretory vesicles to the plasma membrane of chromaffin cells
Wen, Peter J., Osborne, Shona L., Zanin, Mark, Low, Pei Ching, Wang, Hai-Tao A., Schoenwaelder, Simone M., Jackson, Shaun P., Wedlich-Soldner, Roland, Vanhaesebroeck, Bart, Keating, Damien J. and Meunier, Frederic A. (2011) Nature Communications, 2 1: 491.1-491.11. doi:10.1038/ncomms1500
Phosphoinositide 3-kinase δ regulates membrane fission of Golgi carriers for selective cytokine secretion
Low, Pei Ching, Misaki, Ryo, Schroder, Kate, Stanley, Amanda C., Sweet, Matthew J., Teasdale, Rohan D., Vanhaesebroeck, Bart, Meunier, Frédéric A., Taguchi, Tomohiko and Stow, Jennifer L. (2010) Journal of Cell Biology, 190 6: 1053-1065. doi:10.1083/jcb.201001028
Inhibition of the PtdIns(5) kinase PIKfyve disrupts intracellular replication of Salmonella
Kerr, Markus C., Wang, Jack T. H., Castro, Natalie A., Hamilton, Nicholas A., Town, Liam, Brown, Darren L., Meunier, Frederic A., Brown, Nat F., Stow, Jennifer L. and Teasdale, Rohan D. (2010) EMBO Journal, 29 8: 1331-1347. doi:10.1038/emboj.2010.28
Abrogating Munc18-1 SNARE complex interaction has limited impact on exocytosis in PC12 cells
Malintan, Nancy, Nguyen, Tam H, Han, Liping, Latham, Catherine F., Osborne, Shona L., Wen, Peter, Lim, Siew, Joo, Tiffany, Sugita, Shuzo, Collins, Brett M. and Meunier, Frederic A. (2009) The Journal of Biological Chemistry, 284 32: 21637-21646. doi:10.1074/jbc.M109.013508
p75 neurotrophin receptor mediates neuronal cell death by activating GIRK channels through phosphatidylinositol 4,5-bisphosphate
Coulson, E. J., May, L. M., Osborne, S. L., Reid, K., Underwood, C. K., Meunier, F. A., Bartlett, P. F. and Sah, P. (2008) Journal of Neuroscience, 28 1: 315-324. doi:10.1523/JNEUROSCI.2699-07.2008
Identification of secretory granule phosphatidylinositol 4,5-bisphosphateinteracting proteins using an affinity pulldown strategy
Osborne, SL, Wallis, TP, Jimenez, JL, Gorman, JJ and Meunier, FA (2007) Molecular & Cellular Proteomics, 6 7: 1158-1169. doi:10.1074/mcp.M600430-MCP200
Engineering stable peptide toxins by means of backbone cyclization: Stabilization of the alpha-conotoxin MII
Clark, R. J., Fischer, H., Dempster, L., Daly, N. L., Rosengren, K. J., Nevin, S. T., Meunier, F. A., Adams, D. J. and Craik, D. J. (2005) Proceedings of The National Academy of Sciences of The United States of America, 102 39: 13767-13772. doi:10.1073/pnas.0504613102
Getting muscles moving again after botulinum toxin: novel therapeutic challenges
Foran, PG, Davletov, B and Meunier, FA (2003) Trends In Molecular Medicine, 9 7: 291-299. doi:10.1016/S1471-4914(03)00113-8
Glycerotoxin from Glycera convoluta stimulates neurosecretion by up-regulating N-type Ca2+ channel activity
Meunier, Frédéric A., Feng, Zhong-Ping, Molgó, Jordi, Zamponi, Gerald W. and Schiavo, Giampietro (2002) The EMBO Journal, 21 24: 6733-6743. doi:10.1093/emboj/cdf677
Journal Articles
Martínez-Mármol, Ramón, Giordano-Santini, Rosina, Kaulich, Eva, Cho, Ann-Na, Przybyla, Magdalena, Riyadh, Md Asrafuzzaman, Robinson, Emilija, Chew, Keng Yih, Amor, Rumelo, Meunier, Frédéric A., Balistreri, Giuseppe, Short, Kirsty R., Ke, Yazi D., Ittner, Lars M., and Hilliard, Massimo A. (2023) SARS-CoV-2 infection and viral fusogens cause neuronal and glial fusion that compromises neuronal activity. Science Advances, 9 (23) eadg2248, 1-15. doi: 10.1126/sciadv.adg2248
Wallis, Tristan P., Jiang, Anmin, Young, Kyle, Hou, Huiyi, Kudo, Kye, McCann, Alex J., Durisic, Nela, Joensuu, Merja, Oelz, Dietmar, Nguyen, Hien, Gormal, Rachel S., and Meunier, Frédéric A. (2023). Super-resolved trajectory-derived nanoclustering analysis using spatiotemporal indexing. Nature Communications, 14 (1) 3353, 1-16. doi: 10.1038/s41467-023-38866-y
Martínez‐Mármol, Ramón, Chai, YeJin, Conroy, Jacinta N., Khan, Zahra, Hong, Seong‐Min, Kim, Seon Beom, Gormal, Rachel S., Lee, Dae Hee, Lee, Jae Kang, Coulson, Elizabeth J., Lee, Mi Kyeong, Kim, Sun Yeou, and Meunier, Frédéric A. (2023). Hericerin derivatives activates a pan‐neurotrophic pathway in central hippocampal neurons converging to ERK1 /2 signaling enhancing spatial memory. Journal of Neurochemistry, 165 (6), 791-808. doi: 10.1111/jnc.15767
Chhabra, Yash, Seiffert, Pernille, Gormal, Rachel S., Vullings, Manon, Lee, Christine Mei Mei, Wallis, Tristan P., Dehkhoda, Farhad, Indrakumar, Sowmya, Jacobsen, Nina L., Lindorff-Larsen, Kresten, Durisic, Nela, Waters, Michael J., Meunier, Frederic A., Kragelund, Birthe B., and Brooks, Andrew J. (2023). Tyrosine kinases compete for growth hormone receptor binding and regulate receptor mobility and degradation. Cell Reports, 42 (5) 112490, 112490. doi: 10.1016/j.celrep.2023.112490
Joensuu, Merja, Syed, Parnayan, Saber, Saber H, Lanoue, Vanessa, Wallis, Tristan P, Rae, James, Blum, Ailisa, Gormal, Rachel S, Small, Christopher, Sanders, Shanley, Jiang, Anmin, Mahrhold, Stefan, Krez, Nadja, Cousin, Michael A, Cooper‐White, Ruby, Cooper‐White, Justin J, Collins, Brett M, Parton, Robert G, Balistreri, Giuseppe, Rummel, Andreas, and Meunier, Frédéric A. (2023). Presynaptic targeting of botulinum neurotoxin type A requires a tripartite PSG‐Syt1 ‐ SV2 plasma membrane nanocluster for synaptic vesicle entry. The EMBO Journal, 42 (13) e112095. doi: 10.15252/embj.2022112095
Bademosi, Adekunle T., Decet, Marianna, Kuenen, Sabine, Calatayud, Carles, Swerts, Jef, Gallego, Sandra F., Schoovaerts, Nils, Karamanou, Spyridoula, Louros, Nikolaos, Martin, Ella, Sibarita, Jean-Baptiste, Vints, Katlijn, Gounko, Natalia V., Meunier, Frédéric A., Economou, Anastassios, Versées, Wim, Rousseau, Frederic, Schymkowitz, Joost, Soukup, Sandra-F. and Verstreken, Patrik (2023).EndophilinA-dependent coupling between activity-induced calcium influx and synaptic autophagy is disrupted by a Parkinson-risk mutation. Neuron. doi: 10.1016/j.neuron.2023.02.001
Martínez‐Mármol, Ramón, Chai, YeJin, Conroy, Jacinta N., Khan, Zahra, Hong, Seong‐Min, Kim, Seon Beom, Gormal, Rachel S., Lee, Dae Hee, Lee, Jae Kang, Coulson, Elizabeth J., Lee, Mi Kyeong, Kim, Sun Yeou and Meunier, Frédéric A. (2023).Hericerin derivatives activates a pan‐neurotrophic pathway in central hippocampal neurons converging to ERK1 /2 signaling enhancing spatial memory. Journal of Neurochemistry. doi: 10.1111/jnc.15767
Bademosi, Adekunle T. and Meunier, Frédéric A (2023).Unveiling the Nanoscale Dynamics of the Exocytic Machinery in Chromaffin Cells with Single-Molecule Imaging. Methods in molecular biology (Clifton, N.J.), 2565, 311-327. doi: 10.1007/978-1-0716-2671-9_21
Chhabra, Yash, Seiffert, Pernille, Gormal, Rachel S., Vullings, Manon, Lee, Christine Mei Mei, Wallis, Tristan P., Dehkhoda, Farhad, Indrakumar, Sowmya, Jacobsen, Nina L., Lindorff-Larsen, Kresten, Durisic, Nela, Waters, Michael J., Meunier, Frederic A., Kragelund, Birthe B. and Brooks, Andrew (2022).Tyrosine kinases compete for growth hormone receptor binding and regulate receptor mobility and degradation. SSRN Electronic Journal. doi: 10.2139/ssrn.4260495
Martínez-Mármol, Ramón, Small, Christopher, Jiang, Anmin, Palliyaguru, Tishila, Wallis, Tristan P., Gormal, Rachel S., Sibarita, Jean-Baptiste, Götz, Jürgen and Meunier, Frédéric A. (2022).Fyn nanoclustering requires switching to an open conformation and is enhanced by FTLD-Tau biomolecular condensates. Molecular Psychiatry, 28 (2), 1-17. doi: 10.1038/s41380-022-01825-y
Gormal, Rachel S. and Meunier, Frédéric A. (2022).Nanoscale organization of the pre-synapse: tracking the neurotransmitter release machinery. Current Opinion in Neurobiology, 75 102576, 1-10. doi: 10.1016/j.conb.2022.102576
Zhu, Yanshan, Chew, Keng Yih, Wu, Melanie, Karawita, Anjana C., McCallum, Georgina, Steele, Lauren E., Yamamoto, Ayaho, Labzin, Larisa I., Yarlagadda, Tejasri, Khromykh, Alexander A., Wang, Xiaohui, Sng, Julian D. J., Stocks, Claudia J., Xia, Yao, Kollmann, Tobias R., Martino, David, Joensuu, Merja, Meunier, Frédéric A., Balistreri, Giuseppe, Bielefeldt-Ohmann, Helle, Bowen, Asha C., Kicic, Anthony, Sly, Peter D., Spann, Kirsten M. and Short, Kirsty R. (2022).Ancestral SARS-CoV-2, but not Omicron, replicates less efficiently in primary pediatric nasal epithelial cells. PLoS Biology, 20 (8) e3001728, e3001728. doi: 10.1371/journal.pbio.3001728
Schenk, Elaine B., Meunier, Frederic A. and Oelz, Dietmar B. (2022).Spatial redistribution of neurosecretory vesicles upon stimulation accelerates their directed transport to the plasma membrane. PLoS One, 17 (3) e0264521, e0264521. doi: 10.1371/journal.pone.0264521
Seebauer, Caroline T., Graus, Matthew S., Huang, Lan, McCann, Alex J., Wylie-Sears, Jill, Fontaine, Frank R., Karnezis, Tara, Zurakowski, David, Staffa, Steven J., Meunier, Frédéric A., Mulliken, John B., Bischoff, Joyce and Francois, Mathias (2022).Non-β-blocker enantiomers of propranolol and atenolol inhibit vasculogenesis in infantile hemangioma. Journal of Clinical Investigation, 132 (3) e151109. doi: 10.1172/jci151109
McCann, Alex J., Lou, Jieqiong, Moustaqil, Mehdi, Graus, Matthew S, Blum, Ailisa, Fontaine, Frank, Liu, Hui, Luu, Winnie, Rudolffi-Soto, Paulina, Koopman, Peter, Sierecki, Emma, Gambin, Yann, Meunier, Frédéric A., Liu, Zhe, Hinde, Elizabeth and Francois, Mathias (2021).A dominant-negative SOX18 mutant disrupts multiple regulatory layers essential to transcription factor activity. Nucleic Acids Research, 49 (19), 10931-10955. doi: 10.1093/nar/gkab820
Pan, Xiaorong, Zhou, Yimin, Hotulainen, Pirta, Meunier, Frédéric A. and Wang, Tong (2021).The axonal radial contractility: structural basis underlying a new form of neural plasticity. BioEssays, 43 (8) 2100033, 1-13. doi: 10.1002/bies.202100033
Wallis, Tristan P., Venkatesh, Bharat G., Narayana, Vinod K., Kvaskoff, David, Ho, Alan, Sullivan, Robert K., Windels, François, Sah, Pankaj and Meunier, Frédéric A. (2021).Saturated free fatty acids and association with memory formation. Nature Communications, 12 (1) 3443, 3443. doi: 10.1038/s41467-021-23840-3
Zhou, Yong, Ariotti, Nicholas, Rae, James, Liang, Hong, Tillu, Vikas, Tee, Shern, Bastiani, Michele, Bademosi, Adekunle T., Collins, Brett M., Meunier, Frederic A., Hancock, John F. and Parton, Robert G. (2021).Caveolin-1 and cavin1 act synergistically to generate a unique lipid environment in caveolae. Journal of Cell Biology, 220 (3) e202005138. doi: 10.1083/jcb.202005138
Gormal, Rachel S., Padmanabhan, Pranesh, Kasula, Ravikiran, Bademosi, Adekunle T., Coakley, Sean, Giacomotto, Jean, Blum, Ailisa, Joensuu, Merja, Wallis, Tristan P., Lo, Harriet P., Budnar, Srikanth, Rae, James, Ferguson, Charles, Bastiani, Michele, Thomas, Walter G., Pardon, Els, Steyaert, Jan, Yap, Alpha S., Goodhill, Geoffrey J., Hilliard, Massimo A., Parton, Robert G. and Meunier, Frédéric A. (2020).Modular transient nanoclustering of activated β2-adrenergic receptors revealed by single-molecule tracking of conformation-specific nanobodies. Proceedings of the National Academy of Sciences of the United States of America, 117 (48), 30476-30487. doi: 10.1073/pnas.2007443117
Cantuti-Castelvetri, Ludovico, Ojha, Ravi, Pedro, Liliana D., Djannatian, Minou, Franz, Jonas, Kuivanen, Suvi, van der Meer, Franziska, Kallio, Katri, Kaya, Tuğberk, Anastasina, Maria, Smura, Teemu, Levanov, Lev, Szirovicza, Leonora, Tobi, Allan, Kallio-Kokko, Hannimari, Österlund, Pamela, Joensuu, Merja, Meunier, Frédéric A., Butcher, Sarah J., Winkler, Martin Sebastian, Mollenhauer, Brit, Helenius, Ari, Gokce, Ozgun, Teesalu, Tambet, Hepojoki, Jussi, Vapalahti, Olli, Stadelmann, Christine, Balistreri, Giuseppe and Simons, Mikael (2020).Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science, 370 (6518) abd2985, 856-860. doi: 10.1126/science.abd2985
Ariotti, Nicholas, Wu, Yeping, Okano, Satomi, Gambin, Yann, Follett, Jordan, Rae, James, Ferguson, Charles, Teasdale, Rohan D., Alexandrov, Kirill, Meunier, Frederic A., Hill, Michelle M. and Parton, Robert G. (2020).An inverted CAV1 (caveolin 1) topology defines novel autophagy-dependent exosome secretion from prostate cancer cells. Autophagy, 17 (9), 1-17. doi: 10.1080/15548627.2020.1820787
Harper, Callista B., Small, Christopher, Davenport, Elizabeth C., Low, Darryl W., Smillie, Karen J., Martínez-Mármol, Ramón, Meunier, Frédéric A. and Cousin, Michael A. (2020).An epilepsy-associated SV2A mutation disrupts Synaptotagmin-1 expression and activity-dependent trafficking. The Journal of Neuroscience, 40 (23), 4586-4595. doi: 10.1523/jneurosci.0210-20.2020
Wang, Tong, Li, Wei, Martin, Sally, Papadopulos, Andreas, Joensuu, Merja, Liu, Chunxia, Jiang, Anmin, Shamsollahi, Golnoosh, Amor, Rumelo, Lanoue, Vanessa, Padmanabhan, Pranesh and Meunier, Frédéric A. (2020).Radial contractility of actomyosin rings facilitates axonal trafficking and structural stability. Journal of Cell Biology, 219 (5) e201902001, 1-S7. doi: 10.1083/jcb.201902001
Joensuu, Merja, Wallis, Tristan P., Saber, Saber H. and Meunier, Frédéric A. (2019).Phospholipases in neuronal function: a role in learning and memory?. Journal of Neurochemistry, 153 (3) jnc.14918, e14918-333. doi: 10.1111/jnc.14918
Brown, Peter, Tan, Aik-Choon, El-Esawi, Mohamed A., Liehr, Thomas, Blanck, Oliver, Gladue, Douglas P., Almeida, Gabriel M. F., Cernava, Tomislav, Sorzano, Carlos O., Yeung, Andy W. K., Engel, Michael S., Chandrasekaran, Arun Richard, Muth, Thilo, Staege, Martin S., Daulatabad, Swapna V., Widera, Darius, Zhang, Junpeng, Meule, Adrian, Honjo, Ken, Pourret, Olivier, Yin, Cong-Cong, Zhang, Zhongheng, Cascella, Marco, Flegel, Willy A., Goodyear, Carl S., van Raaij, Mark J., Bukowy-Bieryllo, Zuzanna, Campana, Luca G., Kurniawan, Nicholas A. ... RELISH Consortium (2019).Large expert-curated database for benchmarking document similarity detection in biomedical literature search. Database-The Journal of Biological Databases and Curation, 2019, 1-67. doi: 10.1093/database/baz085
Martínez-Mármol, Ramón, Mohannak, Nika, Qian, Lei, Wang, Tong, Gormal, Rachel S, Ruitenberg, Marc J, Vanhaesebroeck, Bart, Coulson, Elizabeth J and Meunier, Frédéric A (2019).p110δ PI 3-kinase inhibition perturbs APP and TNFα trafficking, reduces plaque burden, dampens neuroinflammation and prevents cognitive decline in an Alzheimer's disease mouse model. Journal of Neuroscience, 39 (40), 7976-7991. doi: 10.1523/JNEUROSCI.0674-19.2019
Lanoue, Vanessa, Chai, Ye Jin, Brouillet, Julie Z., Weckhuysen, Sarah, Palmer, Elizabeth E., Collins, Brett M. and Meunier, Frederic A. (2019).STXBP1 encephalopathy: connecting neurodevelopmental disorders with α-synucleinopathies?. Neurology, 93 (3), 114-123. doi: 10.1212/WNL.0000000000007786
Padmanabhan, Pranesh, Martínez-Mármol, Ramón, Xia, Di, Götz, Jürgen and Meunier, Frédéric A. (2019).Frontotemporal dementia mutant Tau promotes aberrant Fyn nanoclustering in hippocampal dendritic spines. eLife, 8 e45040. doi: 10.7554/elife.45040
Brennan, Faith H., Jogia, Trisha, Gillespie, Ellen R., Blomster, Linda V., Li, Xaria X., Nowlan, Bianca, Williams, Gail M., Jacobson, Esther, Osborne, Geoff W., Meunier, Frederic A., Taylor, Stephen M., Campbell, Kate E., MacDonald, Kelli P.A., Levesque, Jean-Pierre, Woodruff, Trent M. and Ruitenberg, Marc J. (2019).Complement receptor C3aR1 controls neutrophil mobilization following spinal cord injury through physiological antagonism of CXCR2. JCI Insight, 4 (9) e98254. doi: 10.1172/jci.insight.98254
Padmanabhan, Pranesh, Bademosi, Adekunle T., Kasula, Ravikiran, Lauwers, Elsa, Verstreken, Patrik and Meunier, Frédéric A. (2019).Need for speed: Super-resolving the dynamic nanoclustering of syntaxin-1 at exocytic fusion sites. Neuropharmacology, 169 107554, 107554. doi: 10.1016/j.neuropharm.2019.02.036
Kasula, Ravikiran, Blum, Ailisa, Salla-Martret, Merce, Chai, Ye Jin, Jiang, Anmin, Wallis, Tristan P., Gormal, Rachel S., Brouillet, Julie Z., Padmanabhan, Pranesh, Weeratunga, Saroja K., Livingstone, Emma K., Mollazade, Mahdie, Joensuu, Merja, Martínez-Mármol, Ramón, Collins, Brett M. and Meunier, Frederic (2019).VAMP2 Binding to Munc18-1 Domain 3a Controls the Nanoscale Reorganization of the Plasma Membrane and Vesicle Interface During Vesicular Priming. SSRN Electronic Journal. doi: 10.2139/ssrn.3362260
Li, Pin, Bademosi, Adekunle T., Luo, Jincai and Meunier, Frederic A. (2018).Actin remodeling in regulated exocytosis: toward a mesoscopic view. Trends in Cell Biology, 28 (9), 685-697. doi: 10.1016/j.tcb.2018.04.004
Chhabra, Y., Wong, H. Y., Nikolajsen, L. F., Steinocher, H., Papadopulos, A., Tunny, K. A., Meunier, F. A., Smith, A. G., Kragelund, B. B., Brooks, A. J. and Waters, M. J. (2018).A growth hormone receptor SNP promotes lung cancer by impairment of SOCS2-mediated degradation. Oncogene, 37 (4), 489-501. doi: 10.1038/onc.2017.352
Bademosi, Adekunle T., Lauwers, Elsa, Amor, Rumelo, Verstreken, Patrik, van Swinderen, Bruno and Meunier, Frédéric A (2018).In vivo single-molecule tracking at the Drosophila presynaptic motor nerve terminal. Journal of Visualized Experiments, 2018 (131) e56952. doi: 10.3791/56952
Bademosi, Adekunle T. , Steeves, James, Karunanithi, Shanker, Zalucki, Oressia H, Gormal, Rachel S, Liu, Shu, Lauwers, Elsa, Verstreken, Patrik, Anggono, Victor, Meunier, Frederic A and van Swinderen, Bruno (2018).Trapping of Syntaxin1a in Presynaptic Nanoclusters by a Clinically Relevant General Anesthetic. Cell reports, 22 (2), 427-440. doi: 10.1016/j.celrep.2017.12.054
Polanco, Juan Carlos, Li, Chuanzhou, Bodea, Liviu-Gabriel, Martinez-Marmol, Ramon, Meunier, Frederic A and Götz, Jürgen (2017).Amyloid-β and tau complexity - towards improved biomarkers and targeted therapies. Nature reviews. Neurology, 14 (1), 22-40. doi: 10.1038/nrneurol.2017.162
Joensuu, Merja, Martinez-Marmol, Ramon, Padmanabhan, Pranesh, Glass, Nick R., Durisic, Nela, Pelekanos, Matthew, Mollazade, Mahdie, Balistreri, Giuseppe, Amor, Rumelo, Cooper-White, Justin J., Goodhill, Geoffrey J. and Meunier, Frederic A. (2017).Visualizing endocytic recycling and trafficking in live neurons by subdiffractional tracking of internalized molecules. Nature Protocols, 12 (12), 2590-2622. doi: 10.1038/nprot.2017.116
May, Linda M., Anggono, Victor, Gooch, Helen M., Jang, Se E., Matusica, Dusan, Kerbler, Georg M., Meunier, Frederic A., Sah, Pankaj and Coulson, Elizabeth J. (2017).G-protein-coupled inwardly rectifying potassium (GIRK) channel activation by the p75 neurotrophin receptor is required for amyloid beta toxicity. Frontiers in Neuroscience, 11 (455) 455. doi: 10.3389/fnins.2017.00455
Tomatis, Vanesa M., Josh, Peter, Papadopulos, Andreas, Gormal, Rachel S., Lanoue, Vanessa, Martin, Sally and Meunier, Frédéric A. (2017).ENA/VASP proteins regulate exocytosis by mediating myosin VI-dependent recruitment of secretory granules to the cortical actin network. Molecular and Cellular Neuroscience, 84, 100-111. doi: 10.1016/j.mcn.2017.07.005
Gormal, R. S., Valmas, N., Fath, T. and Meunier, F. A. (2017).A role for tropomyosins in activity-dependent bulk endocytosis?. Molecular and Cellular Neuroscience, 84, 112-118. doi: 10.1016/j.mcn.2017.04.003
Vanhauwaert, Roeland, Kuenen, Sabine, Masius, Roy, Bademosi, Adekunle, Manetsberger, Julia, Schoovaerts, Nils, Bounti, Laura, Gontcharenko, Serguei, Swerts, Jef, Vilain, Sven, Picillo, Marina, Barone, Paolo, Munshi, Shashini T., de Vrij, Femke M. S., Kushner, Steven A., Gounko, Natalia V., Mandemakers, Wim, Bonifati, Vincenzo, Meunier, Frederic A., Soukup, Sandra‐Fausia and Verstreken, Patrik (2017).The SAC1 domain in synaptojanin is required for autophagosome maturation at presynaptic terminals. EMBO Journal, 36 (10), 1392-1411. doi: 10.15252/embj.201695773
Richter, Sandy, Helm, Conrad, Meunier, Frederic A., Hering, Lars, Campbell, Lahcen I., Drukewitz, Stephan H., Undheim, Eivind A. B., Jenner, Ronald A., Schiavo, Giampietro and Bleidorn, Christoph (2017).Comparative analyses of glycerotoxin expression unveil a novel structural organization of the bloodworm venom system. BMC Evolutionary Biology, 17 (1) 64, 64. doi: 10.1186/s12862-017-0904-4
Bademosi, Adekunle T., Lauwers, Elsa, Padmanabhan, Pranesh, Odierna, Lorenzo, Chai, Ye Jin, Papadopulos, Andreas, Goodhill, Geoffrey J., Verstreken, Patrik, van Swinderen, Bruno and Meunier, Frédéric A (2017).Erratum: In vivo single-molecule imaging of syntaxin1A reveals polyphosphoinositide- and activity-dependent trapping in presynaptic nanoclusters. Nature Communications, 8 (1) 14492. doi: 10.1038/ncomms14492
Bademosi, Adekunle T., Lauwers, Elsa, Padmanabhan, Pranesh, Odierna, Lorenzo, Chai, Ye Jin, Papadopulos, Andreas, Goodhill, Geoffrey J., Verstreken, Patrik, Van Swinderen, Bruno and Meunier, Frederic A. (2016).In vivo single-molecule imaging of syntaxin1A reveals polyphosphoinositide- and activity-dependent trapping in presynaptic nanoclusters. Nature Communications, 7 (1) 13660, 13660. doi: 10.1038/ncomms13660
Joensuu, Merja, Padmanabhan, Pranesh, Durisic, Nela, Bademosi, Adekunle T. D., Cooper-Williams, Elizabeth, Morrow, Isabel C., Harper, Callista B., Jung, WooRam, Parton, Robert G., Goodhill, Geoffrey J., Papadopulos, Andreas and Meunier, Frederic A. (2016).Subdiffractional tracking of internalized molecules reveals heterogeneous motion states of synaptic vesicles. Journal of Cell Biology, 215 (2), 277-292. doi: 10.1083/jcb.201604001
Wang, Tong, Martin, Sally, Nguyen, Tam H., Harper, Callista B., Gormal, Rachel S., Martinez-Marmol, Ramon, Karunanithi, Shanker, Coulson, Elizabeth J., Glass, Nick R., Cooper-White, Justin J., Van Swinderen, Bruno and Meunier, Frederic A. (2016).Flux of signalling endosomes undergoing axonal retrograde transport is encoded by presynaptic activity and TrkB. Nature Communications, 7 (1) 12976, 12976. doi: 10.1038/ncomms12976
Kasula, Ravikiran, Chai, Ye Jin, Bademosi, Adekunle T., Harper, Callista B., Gormal, Rachel S., Morrow, Isabel C., Hosy, Eric, Collins, Brett M., Choquet, Daniel, Papadopulos, Andreas and Meunier, Frederic A. (2016).The Munc18-1 domain 3a hinge-loop controls syntaxin-1A nanodomain assembly and engagement with the SNARE complex during secretory vesicle priming. The Journal of Cell Biology, 214 (7) 847, 847-858. doi: 10.1083/jcb.201508118
Chai, Ye Jin, Sierecki, Emma, Tomatis, Vanesa M., Gormal, Rachel S., Giles, Nichole, Morrow, Isabel C., Xia, Di, Götz, Jürgen, Parton, Robert G., Collins, Brett M., Gambin, Yann and Meunier, Frédéric A. (2016).Munc18-1 is a molecular chaperone for α-synuclein, controlling its self-replicating aggregation. The Journal of Cell Biology, 214 (6) 705, 705-718. doi: 10.1083/jcb.201512016
Meunier, Frederic A. and Gutierrez, Luis M. (2016).Caotivating new roles of F-actin cortex in exocytosis and bulk endocytosis in neurosecretory cells. Trends in Neurosciences, 39 (9), 605-613. doi: 10.1016/j.tins.2016.07.003
Meunier, Frederic A. and Keating, Damien J. (2016).The 18th International Symposia on Chromaffin Cell Biology. Journal of Neurochemistry, 137 (6), 847-848. doi: 10.1111/jnc.13631
Harper, Callista B., Papadopulos, Andreas, Martin, Sally, Matthews, Daniel R., Morgan, Garry P., Nguyen, Tam H., Wang, Tong, Nair, Deepak, Choquet, Daniel and Meunier, Frederic A. (2016).Botulinum neurotoxin type-A enters a non-recycling pool of synaptic vesicles. Scientific Reports, 6 (6) 19654, 19654.1-19654.13. doi: 10.1038/srep19654
Narayana, Vinod K., Tomatis, Vanesa M., Wang, Tong, Kvaskoff, David and Meunier, Frederic A. (2015).Profiling of free fatty acids using stable isotope tagging uncovers a role for saturated fatty acids in neuroexocytosis. Chemistry and Biology, 22 (11), 1552-1561. doi: 10.1016/j.chembiol.2015.09.010
Jackson, J., Papadopulos, A., Meunier, F. A., McCluskey, A., Robinson, P. J. and Keating, D. J. (2015).Small molecules demonstrate the role of dynamin as a bi-directional regulator of the exocytosis fusion pore and vesicle release. Molecular Psychiatry, 20 (7), 810-819. doi: 10.1038/mp.2015.56
Wang, Tong, Martin, Sally, Papadopulos, Andreas, Harper, Callista B., Mavlyutov, Timur A., Niranjan, Dhevahi, Glass, Nick R., Cooper-White, Justin J., Sibarita, Jean-Baptiste, Choquet, Daniel, Davletov, Bazbek and Meunier, Frederic A. (2015).Control of autophagosome axonal retrograde flux by presynaptic activity unveiled using botulinum neurotoxin type A. Journal of Neuroscience, 35 (15), 6179-6194. doi: 10.1523/JNEUROSCI.3757-14.2015
Papadopulos, Andreas, Gomez, Guillermo A., Martin, Sally, Jackson, Jade, Gormal, Rachel S., Keating, Damien J., Yap, Alpha S. and Meunier, Frederic A. (2015).Activity-driven relaxation of the cortical actomyosin II network synchronizes Munc18-1-dependent neurosecretory vesicle docking. Nature Communications, 6 (6297) 6297, 1-11. doi: 10.1038/ncomms7297
Gormal, Rachel S, Nguyen, Tam H, Martin, Sally, Papadopulos, Andreas and Meunier, Frederic A (2015).An acto-myosin II constricting ring initiates the fission of activity-dependent bulk endosomes in neurosecretory cells. Journal of Neuroscience, 35 (4), 1380-1389. doi: 10.1523/JNEUROSCI.3228-14.2015
Lin, Min-Hsuan, Sivakumaran, Haran, Jones, Alun, Li, Dongsheng, Harper, Callista, Wei, Ting, Jin, Hongping, Rustanti, Lina, Meunier, Frederic A., Spann, Kirsten and Harrich, David (2014).A HIV-1 Tat mutant protein disrupts HIV-1 Rev function by targeting the DEAD-box RNA helicase DDX1. Retrovirology, 11 (1) 9, 121.1-121.16. doi: 10.1186/s12977-014-0121-9
Martin, Sally, Papadopulos, Andreas, Tomatis, Vanesa M., Sierecki, Emma, Malintan, Nancy T., Gormal, Rachel S., Giles, Nichole, Johnston, Wayne A., Alexandrov, Kirill, Gambin, Yann, Collins, Brett M. and Meunier, Frederic A. (2014).Increased polyubiquitination and proteasomal degradation of a Munc18-1 disease-linked mutant causes temperature-sensitive defect in exocytosis. Cell Reports, 9 (1), 206-218. doi: 10.1016/j.celrep.2014.08.059
Brooks, Kelly, Ranall, Max, Spoerri, Loredana, Stevenson, Alex, Gunasingh, Gency, Pavey, Sandra, Meunier, Fred, Gonda, Thomas J. and Gabrielli, Brian (2014).Decatenation checkpoint defective melanomas are dependent on PI3K for survival. Pigment Cell and Melanoma Research, 27 (5), 813-821. doi: 10.1111/pcmr.12268
Harper, Callista B., Bademosi, Adekunle T., Coulson, Elizabeth J. and Meunier, Frederic A. (2014).A role for SNAREs in neuronal survival?. Journal of Neurochemistry, 129 (5), 753-755. doi: 10.1111/jnc.12699
Nguyen, Tam H., Qiu, XuFeng, Sun, Jian and Meunier, Frederic A. (2014).Bulk endocytosis at neuronal synapses. Science China Life Sciences, 57 (4), 378-383. doi: 10.1007/s11427-014-4636-z
Low, Pei Ching, Manzanero, Silvia, Mohannak, Nika, Narayana, Vinod K., Nguyen, Tam H., Kvaskoff, David, Brennan, Faith H., Ruitenberg, Marc J., Gelderblom, Mathias, Magnus, Tim, Kim, Hyun Ah, Broughton, Brad R. S., Sobey, Christopher G., Vanhaesebroeck, Bart, Stow, Jennifer L., Arumugam, Thiruma V. and Meunier, Frédéric A. (2014).PI3K delta inhibition reduces TNF secretion and neuroinflammation in a mouse cerebral stroke model. Nature Communications, 5 (3450) 3450, 1-12. doi: 10.1038/ncomms4450
Maucort, Guillaume, Kasula, Ravikiran, Papadopulos, Andreas, Nieminen, Timo A., Rubinsztein-Dunlop, Halina and Meunier, Frederic A. (2014).Mapping Organelle Motion Reveals a Vesicular Conveyor Belt Spatially Replenishing Secretory Vesicles in Stimulated Chromaffin Cells. PLoS One, 9 (1) e87242, e87242. doi: 10.1371/journal.pone.0087242
McCluskey, Adam, Daniel, James A., Hadzic, Gordana, Chau, Ngoc, Clayton, Emma L., Mariana, Anna, Whiting, Ainslie, Gorgani, Nick N., Lloyd, Jonathan, Quan, Annie, Moshkanbaryans, Lia, Krishnan, Sai, Perera, Swetha, Chircop, Megan, von Kleist, Lisa, McGeachie, Andrew B., Howes, Mark T., Parton, Robert G., Campbell, Michael, Sakoff, Jennette A., Wang, Xuefeng, Sun, Jian-Yuan, Robertson, Mark J., Deane, Fiona M., Nguyen, Tam H., Meunier, Frederic A., Cousin, Michael A. and Robinson, Phillip J. (2013).Building a better dynasore: the Dyngo compounds potently inhibit dynamin and endocytosis. Traffic, 14 (12), 1272-1289. doi: 10.1111/tra.12119
Papadopulos, Andreas, Tomatis, Vanesa, Kasula, Ravikiran and Meunier, Frederic A. (2013).The cortical acto-myosin network: from diffusion barrier to functional gateway in the transport of neurosecretory vesicles to the plasma membrane. Frontiers in Endocrinology, 4 (153), 153.1-153.11. doi: 10.3389/fendo.2013.00153
Ferrari, Enrico, Gu, Chunjing, Niranjan, Dhevahi, Restani, Laura, Rasetti-Escargueil, Christine, Obara, Ilona, Geranton, Sandrine M., Arsenault, Jason, Goetze, Tom A., Harper, Callista B., Nguyen, Tam H., Maywood, Elizabeth, O'Brien, John, Schiavo, Giampietro, Wheeler, Daniel W., Meunier, Frederic A., Hastings, Michael, Edwardson, J. Michael, Sesardic, Dorothea, Caleo, Matteo, Hunt, Stephen P. and Davletov, Bazbek (2013).Synthetic self-assembling clostridial chimera for modulation of sensory functions. Bioconjugate Chemistry, 24 (10), 1750-1759. doi: 10.1021/bc4003103
Martin, Sally, Tomatis, Vanesa M., Papadopulos, Andreas, Christie, Michelle P., Malintan, Nancy T., Gormal, Rachel S., Sugita, Shuzo, Martin, Jennifer L., Collins, Brett M. and Meunier, Frederic A. (2013).The Munc18-1 domain 3a loop is essential for neuroexocytosis but not for syntaxin-1A transport to the plasma membrane. Journal of Cell Science, 126 (11), 2353-2360. doi: 10.1242/jcs.126813
Martin, Sally, Harper, Callista B., May, Linda M., Coulson, Elizabeth J., Meunier, Frederic A. and Osborne, Shona L. (2013).Inhibition of PIKfyve by YM-201636 Dysregulates Autophagy and Leads to Apoptosis-Independent Neuronal Cell Death. PLoS One, 8 (3) e60152, e60152.1-e60152.14. doi: 10.1371/journal.pone.0060152
Tomatis, Vanesa M., Papadopulos, Andreas, Malintan, Nancy T., Martin, Sally, Wallis, Tristan, Gormal, Rachel S., Kendrick-Jones, John, Buss, Folma and Meunier, Frederic A. (2013).Myosin VI small insert isoform maintains exocytosis by tethering secretory granules to the cortical actin. Journal of Cell Biology, 200 (3), 301-320. doi: 10.1083/jcb.201204092
Harper, Callista B., Popoff, Michel R., McCluskey, Adam, Robinson, Phillip J. and Meunier, Frederic A. (2013).Targeting membrane trafficking in infection prophylaxis: dynamin inhibitors. Trends in Cell Biology, 23 (2), 90-101. doi: 10.1016/j.tcb.2012.10.007
Papadopulos, Andreas, Martin, Sally, Tomatis, Vanesa M., Gormal, Rachel S. and Meunier, Frederic A. (2013).Secretagogue stimulation of neurosecretory cells elicits filopodial extensions uncovering new functional release sites. Journal of Neuroscience, 33 (49), 19143-19153. doi: 10.1523/JNEUROSCI.2634-13.2013
Nguyen, Tam H., Maucort, Guillaume, Sullivan, Robert K. P., Schenning, Mitja, Lavidis, Nickolas A., McCluskey, Adam, Robinson, Phillip J. and Meunier, Frederic A. (2012).Actin- and dynamin-dependent maturation of bulk endocytosis restores neurotransmission following synaptic depletion. PLoS One, 7 (5 Article No.e36913) e36913, e36913. doi: 10.1371/journal.pone.0036913
Han, Gayoung A., Malintan, Nancy T., Saw, Ner Mu Nar, Li, Lijun, Han, Liping, Meunier, Frederic A., Collins, Brett M. and Sugita, Shuzo (2011).Munc18-1 domain-1 controls vesicle docking and secretion by interacting with syntaxin-1 and chaperoning it to the plasma membrane. Molecular Biology of The Cell, 22 (21), 4134-4149. doi: 10.1091/mbc.E11-02-0135
Wen, Peter J., Osborne, Shona L., Zanin, Mark, Low, Pei Ching, Wang, Hai-Tao A., Schoenwaelder, Simone M., Jackson, Shaun P., Wedlich-Soldner, Roland, Vanhaesebroeck, Bart, Keating, Damien J. and Meunier, Frederic A. (2011).Phosphatidylinositol(4,5)bisphosphate coordinates actin-mediated mobilization and translocation of secretory vesicles to the plasma membrane of chromaffin cells. Nature Communications, 2 (1) 757, 491.1-491.11. doi: 10.1038/ncomms1500
Harper, Callista B., Martin, Sally, Nguyen, Tam H., Daniels, Shari J., Lavidis, Nickolas A., Popoff, Michel R., Hadzic, Gordana, Mariana, Anna, Chau, Ngoc, McCluskey, Adam, Robinson, Phillip J. and Meunier, Frederic A. (2011).Dynamin inhibition blocks botulinum neurotoxin type A endocytosis in neurons and delays botulism. Journal of Biological Chemistry, 286 (41), 35966-35976. doi: 10.1074/jbc.M111.283879
Wen, Peter, Osborne, Shona L. and Meunier, Frederic A. (2011).Dynamic control of neuroexocytosis by phosphoinositides in health and disease. Progress in Lipid Research, 50 (1), 52-61. doi: 10.1016/j.plipres.2010.08.001
Nguyen-Huu, Truong D., Mattei, César, Wen, Peter J., Bourdelais, Andrea J., Lewis, Richard J., Benoit, Evelyne, Baden, Daniel G., Molgó, Jordi and Meunier, Frédéric A. (2010).Ciguatoxin-induced catecholamine secretion in bovine chromaffin cells: Mechanism of action and reversible inhibition by brevenal. Toxicon, 56 (5), 792-796. doi: 10.1016/j.toxicon.2009.08.002
Han, GA, Malintan, NT, Collins, BM, Meunier, FA and Sugita, S (2010).Munc18-1 as a key regulator of neurosecretion. Journal of Neurochemistry, 115 (1), 1-10. doi: 10.1111/j.1471-4159.2010.06900.x
Low, Pei Ching, Misaki, Ryo, Schroder, Kate, Stanley, Amanda C., Sweet, Matthew J., Teasdale, Rohan D., Vanhaesebroeck, Bart, Meunier, Frédéric A., Taguchi, Tomohiko and Stow, Jennifer L. (2010).Phosphoinositide 3-kinase δ regulates membrane fission of Golgi carriers for selective cytokine secretion. Journal of Cell Biology, 190 (6), 1053-1065. doi: 10.1083/jcb.201001028
Anderson, Regan J., Osborne, Shona L., Meunier, Frederic A and Painter, Gavin F. (2010).Regioselective Approach to Phosphatidylinositol 3,5-Bisphosphates: Syntheses of the Native Phospholipid and Biotinylated Short-Chain Derivative. Journal of Organic Chemistry, 75 (11), 3541-3551. doi: 10.1021/jo100393c
Meunier, Frederic A., Nguyen, Tam H., Colasante, Cesare, Luo, Fujun, Sullivan, Robert K. P., Lavidis, Nicholas A., Molgo, Jordi, Meriney, Stephen D. and Schiavo, Giampietro (2010).Sustained synaptic-vesicle recycling by bulk endocytosis contributes to the maintenance of high-rate neurotransmitter release stimulated by glycerotoxin. Journal of Cell Science, 123 (7), 1131-1140. doi: 10.1242/jcs.049296
Kerr, Markus C., Wang, Jack T. H., Castro, Natalie A., Hamilton, Nicholas A., Town, Liam, Brown, Darren L., Meunier, Frederic A., Brown, Nat F., Stow, Jennifer L. and Teasdale, Rohan D. (2010).Inhibition of the PtdIns(5) kinase PIKfyve disrupts intracellular replication of Salmonella. EMBO Journal, 29 (8), 1331-1347. doi: 10.1038/emboj.2010.28
Han, Liping, Jiang, Tiandan, Han, Gayoung A., Malintan, Nancy T., Xie, Li., Wang, Li., Tse, Frederick W., Gaisano, Herbert Y., Collins, Brett M., Meunier, Frederic A. and Sugita, Shuzo (2009).Rescue of Munc18-1 and -2 double knockdown reveals the essential functions of interaction between Munc18 and closed syntaxin in PC12 cells. Molecular Biology of the Cell, 20 (23), 4962-4975. doi: 10.1091/mbc.E09-08-0712
Malintan, Nancy, Nguyen, Tam H, Han, Liping, Latham, Catherine F., Osborne, Shona L., Wen, Peter, Lim, Siew, Joo, Tiffany, Sugita, Shuzo, Collins, Brett M. and Meunier, Frederic A. (2009).Abrogating Munc18-1 SNARE complex interaction has limited impact on exocytosis in PC12 cells. The Journal of Biological Chemistry, 284 (32), 21637-21646. doi: 10.1074/jbc.M109.013508
Wen, Peter, Osborne, Shona L., Morrow, Isabel C., Parton, Robert G., Domin, Jan and Meunier, Frederic A. (2008).Ca2+-regulated pool of phosphatidylinositol-3-phosphate produced by phosphatidylinositol 3-kinase C2α on neurosecretory vesicles. Molecular Biology of the Cell, 19 (12), 5593-5603. doi: 10.1091/mbc.E08-06-0595
Mattei, C, Wen, PJ, Nguyen-Huu, TD, Alvarez, M, Benoit, E, Bourdelais, AJ, Lewis, RJ, Baden, DG, Molgo, J and Meunier, FA (2008).Brevenal Inhibits Pacific Ciguatoxin-1B-Induced Neurosecretion from Bovine Chromaffin Cells. PLoS One, 3 (10) e3448, 1-16. doi: 10.1371/journal.pone.0003448
Osborne, Shona L., Wen, Peter J., Boucheron, Christine, Nguyen, Hao N., Hayakawa, Masahiko, Kaizawa, Hiroyuki, Parker, Peter J., Vitale, Nicolas and Meunier, Frederic A. (2008).PIKfyve Negatively Regulates Exocytosis in Neurosecretory Cells. Journal of Biological Chemistry, 283 (5), 2804-2813. doi: 10.1074/jbc.M704856200
Coulson, E. J., May, L. M., Osborne, S. L., Reid, K., Underwood, C. K., Meunier, F. A., Bartlett, P. F. and Sah, P. (2008).p75 neurotrophin receptor mediates neuronal cell death by activating GIRK channels through phosphatidylinositol 4,5-bisphosphate. Journal of Neuroscience, 28 (1), 315-324. doi: 10.1523/JNEUROSCI.2699-07.2008
Latham, C. F., Osborne, S. L., Cryle, M. J. and Meunier, F. A. (2007).Arachidonic acid potentiates exocytosis and allows neuronal SNARE complex to interact with Munc18a. Journal of Neurochemistry, 100 (6), 1543-1554. doi: 10.1111/j.1471-4159.2006.04286.x
Osborne, SL, Latham, CF, Wen, PJ, Cavaignac, S, Fanning, J, Foran, PG and Meunier, FA (2007).The janus faces of botulinum neurotoxin: Sensational medicine and deadly biological weapon. Journal of Neuroscience Research, 85 (6), 1149-1158. doi: 10.1002/jnr.21171
Osborne, SL, Wallis, TP, Jimenez, JL, Gorman, JJ and Meunier, FA (2007).Identification of secretory granule phosphatidylinositol 4,5-bisphosphateinteracting proteins using an affinity pulldown strategy. Molecular and Cellular Proteomics, 6 (7), 1158-1169. doi: 10.1074/mcp.M600430-MCP200
Latham, CF and Meunier, FA (2007).Munc 18a: Munc-y business in mediating exocytosis. International Journal of Biochemistry & Cell Biology, 39 (9), 1576-1581. doi: 10.1016/j.biocel.2006.11.015
Schenning, Mitja, Proctor, Dustin T., Ragnarsson, Lotten, Barbier, Julien, Lavidis, Nickolas A., Molgo, Jordi J., Zamponi, Gerald W., Schiavo, Giampietro and Meunier, Frederic A. (2006).Glycerotoxin stimulates neurotransmitter release from N-type Ca2+ channel expressing neurons. Journal of Neurochemistry, 98 (3), 894-904. doi: 10.1111/j.1471-4159.2006.03938.x
Sim, Alistair T.R., Herd, Lynn, Proctor, Dustin T., Baldwin, Monique L., Meunier, Frederic A. and Rostas, John A.P (2006).High throughput analysis of endogenous glutamate release using a fluorescence plate reader. Journal of Neuroscience Methods, 153 (1), 43-47. doi: 10.1016/j.jneumeth.2005.10.004
Osborne, Shona L., Wen, Peter J. and Meunier, Frederic A. (2006).Phosphoinositide regulation of neuroexocytosis: adding to the complexity. Journal of Neurochemistry, 98 (2), 336-342. doi: 10.1111/j.1471-4159.2006.03892.x
Meunier, Frederic A., Osborne, Shona L., Hammond, Gerald R.V., Cooke, Frank T., Parker, Peter J., Domin, Jan and Schiavo, Giampietro (2005).Phosphatidylinositol 3-kinase C2 alpha is essential for ATP-dependent priming of neurosecretory granule exocytosis. Molecular Biology of The Cell, 16 (10), 4841-4851. doi: 10.1091/mbc.E05-02-0171
Clark, R. J., Fischer, H., Dempster, L., Daly, N. L., Rosengren, K. J., Nevin, S. T., Meunier, F. A., Adams, D. J. and Craik, D. J. (2005).Engineering stable peptide toxins by means of backbone cyclization: Stabilization of the alpha-conotoxin MII. Proceedings of The National Academy of Sciences of The United States of America, 102 (39), 13767-13772. doi: 10.1073/pnas.0504613102
Rickman, Colin, Archer, Deborah A., Meunier, Frederic A., Craxton, Molly, Fukuda, Mitsunori, Burgoyne, Robert D. and Davletov, Bazbek (2004).Synaptotagmin interaction with the syntaxin/SNAP-25 dimer is mediated by an evolutionarily conserved motif and is sensitive to inositol hexakisphosphate. Journal of Biological Chemistry, 279 (13), 12574-12579. doi: 10.1074/jbc.M310710200
Rickman, Colin, Meunier, Frederic A., Binz, Thomas and Davletov, Bazbek (2004).High affinity interaction of syntaxin and SNAP-25 on the plasma membrane is abolished by botulinum toxin E. Journal of Biological Chemistry, 279 (1), 644-651. doi: 10.1074/jbc.M310879200
Meunier, Frederic, Lisk, Godfrey, Sesardic, Dorothea and Dolly, J. Oliver (2003).Dynamics of motor nerve terminal remodeling unveiled using SNARE-cleaving botulinum toxins: the extent and duration are dictated by the sites of SNAP-25 truncation. Molecular and Cellular Neuroscience, 22 (4), 454-466. doi: 10.1016/S1044-7431(02)00016-7
Jimenez, J. L., Smith, G. R., Contreras-Moreira, B., Sgouros, J. G., Meunier, F. A., Bates, P. A. and Schiavo, G. (2003).Functional recycling of C2 domains throughout evolution: A comparative study of synaptotagmin, protein kinase C and phospholipase C by sequence, structural and modelling approaches. Journal of Molecular Biology, 333 (3), 621-639. doi: 10.1016/j.jmb.2003.08.052
Foran, PG, Davletov, B and Meunier, FA (2003).Getting muscles moving again after botulinum toxin: novel therapeutic challenges. Trends In Molecular Medicine, 9 (7), 291-299. doi: 10.1016/S1471-4914(03)00113-8
Meunier, FA, Schiavo, G and Molgo, J (2002).Botulinum neurotoxins: from paralysis to recovery of functional neuromuscular transmission. Journal of Physiology-paris, 96 (1-2), 105-113. doi: 10.1016/S0928-4257(01)00086-9
Verastegui, C., Lalli, G., Bohnert, S., Meunier, F. A. and Schiavo, G. (2002).Clostridial neurotoxins. Journal of Toxicology-toxin Reviews, 21 (3), 203-227. doi: 10.1081/TXR-120014404
Benoit, Evelyne, Mattei, Cesar, Ouanounou, Gilles, Meunier, Frederic A., Suput, Dusan, Le Gall, Frederic, Marquais, Michel, Dechraoui, Marie Y. and Molgo, Jordi (2002).Ionic mechanisms involved in the nodal swelling of myelinated axons caused by marine toxins. Cellular & Molecular Biology Letters, 7 (2), 317-321.
Meunier, Frédéric A., Feng, Zhong-Ping, Molgó, Jordi, Zamponi, Gerald W. and Schiavo, Giampietro (2002).Glycerotoxin from Glycera convoluta stimulates neurosecretion by up-regulating N-type Ca2+ channel activity. The EMBO Journal, 21 (24), 6733-6743. doi: 10.1093/emboj/cdf677
Osborne, Shona L., Meunier, Frédéric A. and Schiavo, Giampietro (2001).Phosphoinositides as key regulators of synaptic function. Neuron, 32 (1), 9-12. doi: 10.1016/S0896-6273(01)00455-X
Volynski, KE, Meunier, FA, Lelianova, VG, Dudina, EE, Volkova, TM, Rahman, MA, Manser, C, Grishin, EV, Dolly, JO, Ashley, RH and Ushkaryov, YA (2000).Latrophilin, neurexin, and their signaling-deficient mutants facilitate α-latrotoxin insertion into membranes but are not involved in pore formation. Journal of Biological Chemistry, 275 (52), 41175-41183. doi: 10.1074/jbc.M005857200
Meunier, FA, Frangez, R, Benoit, E, Ouanounou, G, Rouzaire-Dubois, B, Suput, D and Molgo, J (2000).Ca2+ and Na+ contribute to the swelling of differentiated neuroblastoma cells induced by equinatoxin-II. Toxicon, 38 (11), 1547-1560. doi: 10.1016/S0041-0101(00)00088-X
Frangež, Robert, Meunier, Frederick, Molgo, Jordi and Suput, Dusan (2000).Equinatoxin II increases intracellular Ca2+ in NG 108–15 cells. Pflugers Archiv European Journal of Physiology, 439 (7), R100-R101. doi: 10.1007/BF03376535
Meunier, FA, Mattei, C, Chameau, P, Lawrence, G, Colasante, C, Kreger, AS, Dolly, JO and Molgo, J (2000).Trachynilysin mediates SNARE-dependent release of catecholamines from chromaffin cells via external and stored Ca2+. Journal of Cell Science, 113 (7), 1119-1125.
Sauviat, MP, Meunier, FA, Kreger, A and Molgo, J (2000).Effects of trachynilysin, a protein isolated from stonefish (Synanceia trachynis) venom, on frog atrial heart muscle. Toxicon, 38 (7), 945-959. doi: 10.1016/S0041-0101(99)00207-X
Ouanounou, G, Mattei, C, Meunier, FA, Kreger, AS and Molgo, J (2000).Trachynilysin, a protein neurotoxin isolated from stonefish (Synanceia trachynis) venom, increases spontaneous quantal acetylcholine release from Torpedo marmorata neuromuscular junctions. Cybium, 24 (3), 149-156.
Shamotienko, O, Akhtar, S, Sidera, C, Meunier, FA, Ink, B, Weir, M and Dolly, JO (1999).Recreation of neuronal Kv1 channel oligomers by expression in mammalian cells using semliki forest virus. Biochemistry, 38 (51), 16766-16776. doi: 10.1021/bi991039n
Mattei, C, Dechraoui, MY, Molgo, J, Meunier, FA, Legrand, AM and Benoit, E (1999).Neurotoxins targetting receptor site 5 of voltage-dependent sodium channels increase the nodal volume of myelinated axons. Journal of Neuroscience Research, 55 (6), 666-673. doi: 10.1002/(SICI)1097-4547(19990315)55:6<666::AID-JNR2>3.0.CO;2-H
Rahman, MA, Ashton, AC, Meunier, FA, Davletov, BA, Dolly, JO and Ushkaryov, YA (1999).Norepinephrine exocytosis stimulated by alpha-latrotoxin requires both external and stored Ca2+ and is mediated by latrophilin, G proteins and phospholipase C. Philosophical Transactions of the Royal Society of London Series B-Biological Sciences, 354 (1381), 379-386. doi: 10.1098/rstb.1999.0390
Davletov, BA, Meunier, FA, Ashton, AC, Matsushita, H, Hirst, WD, Lelianova, VG, Wilkin, GP, Dolly, JO and Ushkaryov, YA (1998).Vesicle exocytosis stimulated by α-latrotoxin is mediated by latrophilin and requires both external and stored Ca2+. EMBO Journal, 17 (14), 3909-3920. doi: 10.1093/emboj/17.14.3909
Cifuentes-Diaz, C, Velasco, E, Meunier, FA, Goudou, D, Belkadi, L, Faille, L, Murawsky, M, Angaut-Petit, D, Molgo, J, Schachner, M, Saga, Y, Aizawa, S and Rieger, F (1998).The peripheral nerve and the neuromuscular junction are affected in the tenascin-C-deficient mouse. Cellular and Molecular Biology, 44 (2), 357-379.
Molgó, Jordi, Meunier, Frédéric A., Colasante, Cesare and Poulain, Bernard (1997).Marine Toxins Affecting Quantal Acetylcholine Release and Transmission at the Vertebrate Neuromuscular Junction. Advances in Organ Biology, 2 (C), 249-284. doi: 10.1016/S1569-2590(08)60189-5
Meunier, FA, Colasante, C and Molgo, J (1997).Sodium-dependent increase in quantal secretion induced by brevetoxin-3 in Ca2+-free medium is associated with depletion of synaptic vesicles and swelling of motor nerve terminals in situ. Neuroscience, 78 (3), 883-893. doi: 10.1016/S0306-4522(96)00568-4
Meunier, FA, Mercado, JA, Molgo, J, Tosteson, TR and deMotta, GE (1997).Selective depolarization of the muscle membrane in frog nerve-muscle preparations by a chromatographically purified extract of the dinoflagellate Ostreopsis lenticularis. British Journal of Pharmacology, 121 (6), 1224-1230. doi: 10.1038/sj.bjp.0701256
Gaudry-Talarmain, YM, Moulian, N, Meunier, FA, Blanchard, B, Angaut-Petit, D, Faille, L and Ducrocq, C (1997).Nitric oxide and peroxynitrite affect differently acetylcholine release, choline acetyltransferase activity, synthesis, and compartmentation of newly formed acetylcholine in Torpedo marmorata synaptosomes. Nitric Oxide - Biology and Chemistry, 1 (4), 330-345. doi: 10.1006/niox.1997.0141
FalkVairant, J, Israel, M, Bruner, J, Stinnakre, J, Meunier, FM, Gaultier, P, Meunier, FA, Lesbats, B, Synguelakis, M, Correges, P and Dunant, Y (1996).Enhancement of quantal transmitter release and mediatophore expression by cyclic AMP in fibroblasts loaded with acetylcholine. Neuroscience, 75 (2), 353-360. doi: 10.1016/0306-4522(96)00260-6
Colasante, C, Meunier, FA, Kreger, AS and Molgo, J (1996).Selective depletion of clear synaptic vesicles and enhanced quantal transmitter release at frog motor nerve endings produced by trachynilysin, a protein toxin isolated from stonefish (Synanceia trachynis) venom. European Journal of Neuroscience, 8 (10), 2149-2156. doi: 10.1111/j.1460-9568.1996.tb00736.x
GaudryTalarmain, YM, Molgo, J, Meunier, FA, Moulian, N and Legrand, AM (1996).Reversed mode Na+-Ca2+ exchange activated by ciguatoxin (CTX-1b) enhances acetylcholine release from Torpedo cholinergic synaptosomes. Sodium-Calcium Exchange, 779, 404-406. doi: 10.1111/j.1749-6632.1996.tb44814.x
Varoqui, H, Meunier, FM, Meunier, FA, Molgo, J, Berrard, S, Cervini, R, Mallet, J, Israel, M and Diebler, MF (1996).Expression of the vesicular acetylcholine transporter in mammalian cells. Cholinergic Mechanisms: From Molecular Biology to Clinical Significance, 109, 83-95.
Conference Papers
Meunier, F. (2019).Unraveling Munc18/Syntaxin1 nanoscale organisation dynamics in health and disease. ISN-ASN Meeting, Montreal, QC, Canada, 4 - 8 August 2019. Chichester, West Sussex, United Kingdom: Wiley-Blackwell Publishing. doi: 10.1111/jnc.14780
Joensuu, Merja, Blum, Ailisa, Mollazade, Mahdie, Lanoue, Vanessa, Padmanabhan, Pranesh, Small, Christopher, Mahrhold, Stefan, Krez, Nadja, Rummel, Andreas and Meunier, Frederic A. (2018).Super-resolving botulinum neurotoxin type A molecules: from surface landing to internalization in synaptic vesicles. TOXINS 2019: Basic Science and Clinical Aspects of Botulinum and Other Neurotoxins, Copenhagen, Denmark, 16-19 January 2019. Kidlington, Oxford, United Kingdom: Pergamon Press. doi: 10.1016/j.toxicon.2018.11.122
Harper, Callista B., Wang, Tong, Joensuu, Merja, Papadopulos, Andreas and Meunier, Frederic A. (2017).Nanoscale imaging of botulinum neurotoxin type a retrograde axonal trafficking. TOXINS: Basic Science and Clinical Aspects of Botulinum and other Neurotoxins, Madrid, Spain, 18-21 January 2017. Oxford, United Kingdom: Pergamon Press. doi: 10.1016/j.toxicon.2016.11.106
Wang, T., Martin, S., Papadopulos, A., Harper, C., Mavlyutov, T., Niranjan, D., Glass, N., Cooper-White, J., Sibarita, J. -B., Choquet, D., Davletov, B. and Meunier, F. (2015).Control of autophagosome axonal retrograde flux by presynaptic activity unveiled using botulinum neurotoxin type-A. 25th Biennial Meeting of the International-Society-for-Neurochemistry Jointly with the 13th Meeting of the Asian-Pacific-Society-for-Neurochemistry in Conjunction with the 35th Meeting of the Australasian-Neuroscience-Society, Cairns Australia, 23-27 August 2015. Hoboken, NJ United States: Wiley-Blackwell.
Low, Pei Ching (Regine), Manzanero, Silvia, Narayana, Vinod K., Nguyen, Tam H., Kvaskoff, David, Brennan, Faith H., Ruitenberg, Marc J., Gelderblom, Mathias, Magnus, Tim, Kim, Hyun Ah, Broughton, Brad R. S., Sobey, Christopher G., Vanhaesebroeck, Bart, Stow, Jennifer L., Arumugam, Thiruma V. and Meunier, Frederic A. (2014).PI3K delta inhibition reduces TNF secretion and neuroinflammation in a mouse cerebral stroke model. 2nd Annual Meeting of the International-Cytokine-and-Interferon-Society (ICIS), Melbourne, Australia, 26-29 October 2014. London, United Kingdom: Academic Press. doi: 10.1016/j.cyto.2014.07.126
Martin, Sally, Harper, Callista B. and Meunier, Frederic A. (2012).Retrograde trafficking at the presynaptic nerve terminal using bacterial toxins. 17th World Congress of the International-Society-on-Toxinology (IST)/Venom Week/4th International Scientific Symposium on All Things Venomous, Honolulu, Hawaii, United States, 8-13 July 2012. Kidlington, Oxford, United Kingdom: Pergamon. doi: 10.1016/j.toxicon.2012.04.130
Chasserot-Golaz, S., Coorssen, J. R., Meunier, F. A. and Vitale, N. (2010).Lipid dynamics in exocytosis. 15th International Symposium on Chromaffin Cell Biology, Merida, Mexico, 12-16 November 2009. New York, NY, U.S.A.: Springer New York LLC. doi: 10.1007/s10571-010-9577-x
Alvarez-Elizondo, Martha B., Roelofs, Susan H., Meunier, Frederic A., Heckenberg, Norman and Rubinsztein-Dunlop, Halina (2008).Noninvasive measurement of intracellular viscoelastic properties. Optical Trapping and Optical Micromanipulation V, San Diego, United States, 10-14 August 2008. Bellingham, WA, United States: S P I E - International Society of Optical Engineering. doi: 10.1117/12.793591
Meunier, FA, Ragnarsson, L, Proctor, D, Feng, ZP, Molgo, J, Zamponi, GW and Schiavo, G (2005).Glycerotoxin and the regulation of neurotransmitter release. 20th Biennial Meeting of the International-Society-for-Neurochemistry/European-Society-for-Neurochemistry, Innsbruck Austria, Aug 21-26, 2005. OXFORD: BLACKWELL PUBLISHING.
Schiavo, G, Meunier, FA, Feng, ZP, Molgo, J and Zamponi, GW (2004).Glycerotoxin stimulates neurosecretion by up-regulating N-type Ca2+ channel activity. 6th Biennial Meeting of the Asian-Pacific-Society-for-Neurochemistry, Hong Kong Peoples R China, Feb 04-07, 2004. OXFORD: BLACKWELL PUBLISHING LTD.
Meunier, F. A., Cooke, F. T., Osborne, S. L., Hammond, G., Domin, J., Parker, P. J. and Schiavo, G. (2004).Class II PI3-kinase C2 alpha is essential for ATP-dependent printing of neurosecretory granule exocytosis. 58th Conference of the Society-of-Nutrition-Physiology, Gottingen, Germany, 9-11 March, 2004. Baltimore, MD: Baltimore, MD.
Schiavo, G, Meunier, FA, Feng, ZP, Zamponi, GW and Molgo, J (2003).Glycerotoxin stimulates neurosecretion by up-regulating N-type calcium channel activity. Joint Meeting of the International-Society-for-Neurochemistry/Asian-Pacific-Society-for-Neurochemistry, Wanchai Peoples R China, Aug 03-08, 2003. OXFORD: BLACKWELL PUBLISHING LTD.
Lisk, G, Meunier, FA, Poran, PO, Mohammed, N, de Puva, A and Dolly, JO (2001).Synapse remodelling after neuroparalysis due to cleavage of SNAREs by botlulinum toxins. CLARE: ELSEVIER SCI IRELAND LTD.
Meunier, FA, Mattei, C, Colasante, C, Chameau, P, Ouanounou, G, Thieffry, M, Ushkaryov, YA, Dolly, JO, Kreger, AS and Molgo, J (2001).Trachynilysin, a stonefish protein that affects neurotransmitter release. OXFORD: BLACKWELL SCIENCE LTD.
de Paiva, A, Meunier, FA, Molgo, J, Aoki, KR and Dolly, JO (1999).Functional repair of motor endplates after botulinum neurotoxin type A poisoning: Biphasic switch of synaptic activity between nerve sprouts and their parent terminals. doi: 10.1073/pnas.96.6.3200
Davletov, BA, Ashton, AC, Meunier, FA, Matsushita, H, Hirst, WD, Dolly, JO and Ushkarvov, YA (1998).alpha-Latrotoxin stimulates vesicle exocytosis via latrophilin- and PLC-mediated coupling of external and stored Ca-2 and induces Ca2+-independent membrane pores. OXFORD: BLACKWELL SCIENCE LTD.
Molgo, J, Meunier, FA and Sellin, LC (1997).Quantal transmitter release at botulinum-treated vertebrate neuromuscular junctions. 11th Meeting of the European-Society-for-Neurochemistry, Groningen Netherlands, Jun 15-20, 1996. NEW YORK: PLENUM PRESS DIV PLENUM PUBLISHING CORP.
Meunier, Frédéric A., Colasante, Cesare, Faille, Lucette, Gastard, Myriam and Molgó, Jordi (1996).Upregulation of calcitonin gene-related peptide at mouse motor nerve terminals poisoned with botulinum type-A toxin.
Molgo, J, Meunier, FA and Sellin, LC (1996).Quantal transmitter release at botulinum-treated vertebrate neuromuscular junctions.. 11th Meeting of the European-Society-for-Neurochemistry, Groningen Netherlands, Jun 15-20, 1996. NEW YORK: PLENUM PRESS DIV PLENUM PUBLISHING CORP.
Varoqui, H., Meunier, F. M., Meunier, F. A., Molgo, J., Berrard, S., Cervini, R., Mallet, J., Israel, M. and Diebler, M. F. (1996).Expression of the vesicular acetylcholine transporter in mammalian cells. Elsevier B.V.
The University of Queensland
- Professor Elizabeth Coulson, QBI
- Professor Jürgen Götz, QBI
- Associate Professor Massimo Hilliard, QBI
- Associate Professor Bruno van Swinderen, QBI
- Associate Professor Brett Collins, IMB
- Professor Jenny Stow, IMB
- Professor Robert Parton, IMB
- Associate Professor Rohan Teasdale, IMB
- Professor Alpha Yap, IMB
- Professor Justin Cooper-White, AIBN
- Associate Professor Peter Noakes, SBMS
- Dr. Marc Ruitenberg, SBMS
National
- Dr. Thomas Fath - Faculty of Medicine, University of New South Wales
- Professor Phil Robinson - Children's Medical Research Institute (CMRI)
- Professor Adam McCluskey - School of Environmental and Life Sciences, University of Newcastle
- Associate Professor Damien Keating - School of Medicine, Flinders University
- Dr. Yann Gambin - European Molecular Biology Laboratory Australia (EMBL), University of New South Wales.
- Dr. Emma Siereki - School of Medical Science, University of New South Wales.
International
- Professor Daniel Choquet - Interdisciplinary Institute for Neuroscience, Bordeaux, France.
- Dr. Giuseppe Balistreri - Department of Biosciences University of Helsinki, Finland.
- Dr. David Kvaskoff - Centre for Biochemistry, University of Heidelberg, Germany.
- Professor Michel R Popoff - Unit for Anaerobic Bacteria and Toxins, Institute Pasteur, Paris, France.
- Professor Jianyuan Sun - The Institute of Biophysics, The Chinese Academy of Science, Beijing, China.
- Professor Shuzo Sugita - Division of Fundamental Neurobiology, Toronto Western Research Institute, Toronto, Canada.
- Professor Britta Brügger - Centre for Biochemistry, University of Heidelberg, Germany.
- Professor Bazbek Davletov - Department of Biomedical Science, The University of Sheffield, England
Super-resolving neurotransmitter release machinery during priming
(2022-2024) ARC Discovery Project
Targeting neuropilin in SARS-CoV-2 neuronal uptake and transport
(2022-2025) NHMRC Ideas Grant
Investigating myristic acid as key mediator of memory via myristoylation
(2022-2025) NHMRC Ideas Grant
Role of Tau and Synapsin in clustering distinct synaptic vesicle pools
(2023-2026) ARC Discovery Project
Single molecule biomolecular condensate analysis in neurons
(2023-2025) NIH
Tau and ITS master regulator Fyn in Neurons
(2017–2019) NHMRC Project Grant
Understanding axonal fusion: an alternative mechanism to repair injured axons.
(2017–2019) NHMRC Project Grant
Unravelling a new fatty acid pathway involved in neuroexocytosis and memory
(2017–2019) NHMRC Project Grant
Unravelling the mechanism coupling synaptic activity with neurotrophin signaling in the nervous system
(2017–2019) NHMRC Project Grant
Unveiling the intra and intermolecular steps underpinning vesicular priming
(2017–2019) ARC Discovery Projects
Embryonic Animals and Tissue Culture
(2016–2017) CNGBio Corp
Unravelling the mechanism of vesicular docking in neurosecretory cells
(2015–2018) ARC Discovery Projects
Vesicular trafficking pathways underpinning neuronal secretion and survival
(2014–2018) NHMRC Research Fellowship (SRFB)
Prospective candidates should be highly motivated and have previous experience in neuroscience, microscopy and/or biophysics and engineering.
Project 1: Use of single molecule imaging to track botulinum neurotoxin interaction with the neuronal membrane
Project 2: Use of single molecule imaging to track epigenetic modifications elicited by synaptic plasticity associated with memory
Project 3: Use of single molecule imaging to uncover the change in presynaptic nanoscale organization occurring during neurotransmitter release (endocytosis)
Project 4: Use of single molecule imaging to uncover the change in presynaptic nanoscale organization occurring during neurotransmitter release (exocytosis)
Project 5: Use of single molecule imaging to uncover the change in Fyn and associated signalling cascade occurring during the development of Alzheimer’s disease
Project 6: Use of single molecule imaging to uncover the change in a-synuclein nanoscale organization in neurons associated with various disease-causing mutations
Research Areas
- Vesicular trafficking in neurons and neurosecretory cells
- Neuroexocytosis
- Neurodegenerative conditions
Podcast
Latest news
-
-
The surprising role of fat in memory formation
18 June 2025 -
New study signals gamechanger for antivirals
26 November 2024