Synaptic Plasticity
The Sah laboratory studies the physiological and molecular mechanisms that underlie learning and memory formation, focussing on the part of the brain called the amygdala.
Synaptic plasticity is fundamental to neural processes underlying learning and memory. Synaptic plasticity is the ability of the brain to adapt new conditions and information. This adaptability is effectively the way that synapses change — becoming stronger or weaker.
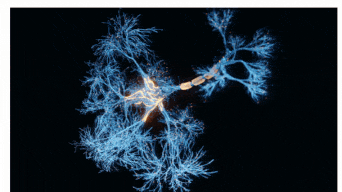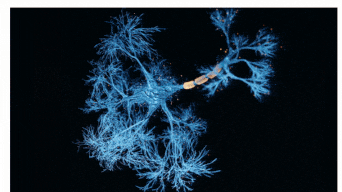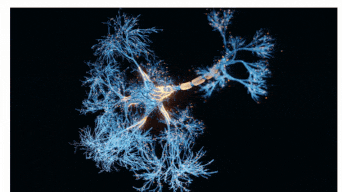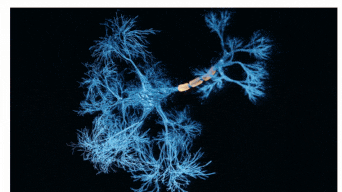
The Sah laboratory uses electrophysiology and molecular techniques, in conjunction with behavioural studies, to understand the neural circuitry that underpins learning and memory formation.
The role of the amygdala in learning and memory
Using animal models, the laboratory focuses on the part of the brain called the amygdala, and an associative learning paradigm called fear conditioning.
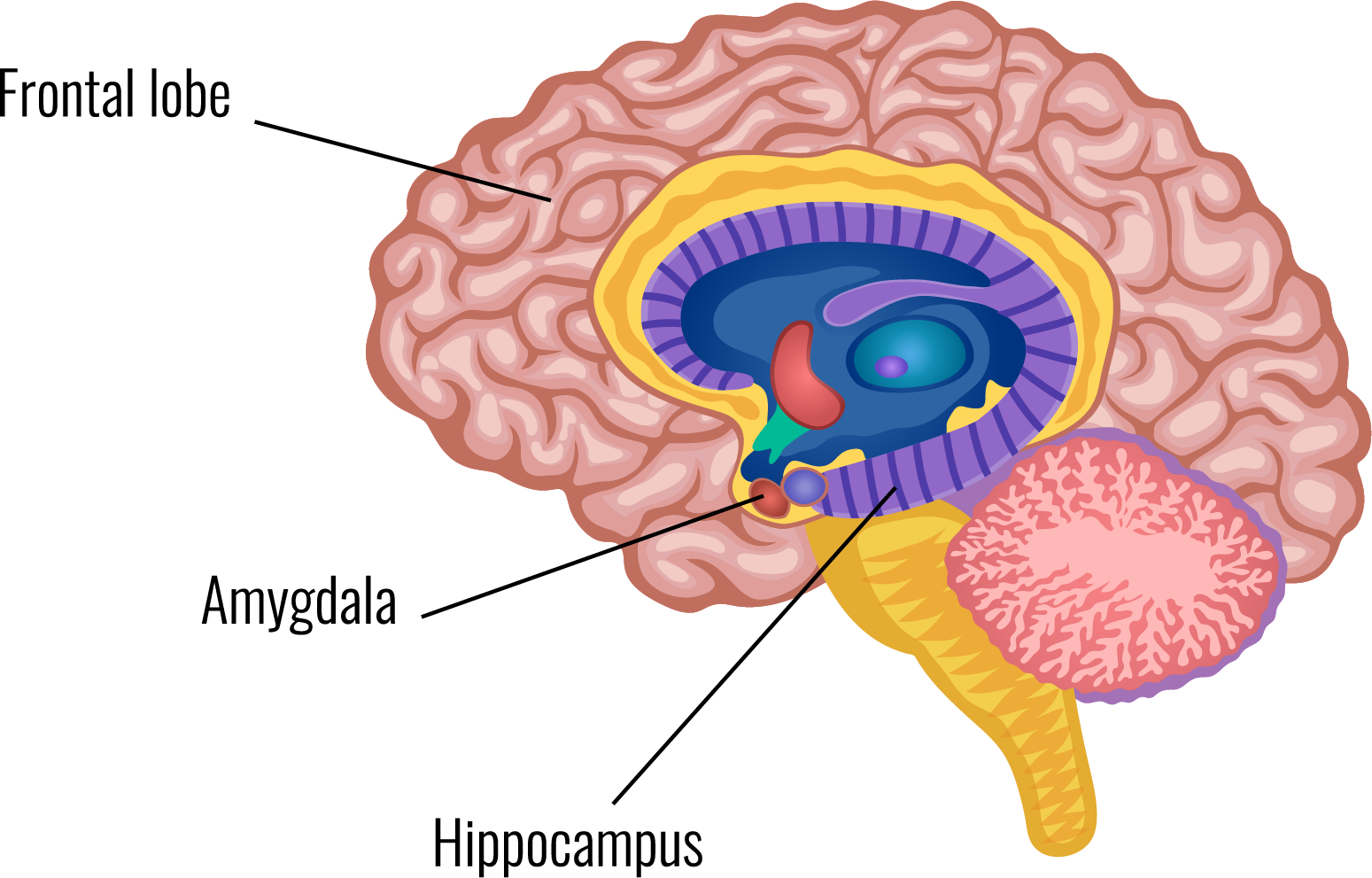
The amygdala is an almond-shaped structure in the brain's temporal lobe. The amygdala attaches emotional significance to memories.
comprised of two amygdalae, one in each cerebral hemisphere
Mapping neural circuits in learning and memory
The Sah group aims to understand how associative learning memories are formed and retrieved to drive appropriate behaviour. They use a technique called optogenetics, an approach that modulates the activity of cells using light, to study neural activity in defined brain regions.
They record electrical activity in acute brain slices to study the neural circuits and the properties of these connections. The group is mapping the circuits that provide sensory and harmful information to the amygdala to better understand the interactions between the amygdala, the prefrontal cortex, and the hippocampus. This information is then used to understand the role of these circuits in memory formation and behaviour.
Deep brain stimulation
Animal studies conducted in the Sah laboratory are complemented by electrophysiological recordings in humans. For these studies, Professor Sah collaborates with Professor Peter Silburn and Dr Terry Coyne, who are part of the Asia–Pacific Centre for Neuromodulation (APCN).
completed their 1000th deep brain stimulation surgery
They study neural activity in the brains of patients undergoing neurosurgery for deep brain stimulation. These recordings reveal the brain activity in a range of movement disorders, such as Parkinson’s disease, essential tremor and Tourette syndrome. In 2016, the group began an Australian-first clinical trial for the treatment of obsessive-compulsive disorder. Results of the trial have demonstrated that deep brain stimulation successfully helps people with severe OCD who have not responded to other treatment.
The clinical trial results are published in the Nature journal Translational Psychiatry.
Professor Sah is interested in the Neuroscience of Learning and how this relates to educational outcomes in classrooms. He is Editor in Chief of the Nature partner journal npj Science of Learning.
Group leader

Professor Pankaj Sah
Institute Director, Queensland Brain Institute
@pankajqbi
+61 7 334 66311
pankaj.sah@uq.edu.au
UQ Researcher Profile
Network activity and the role of NMDA receptors in associative learning
(2022–2025) ARC Discovery Projects
Rotary International District Human Brain Global Grant PhD Scholarship
(2021–2025) Rotary International District 9640 Ltd
Unravelling amygdala-hippocampus neural circuitry of anxiety: Role of adult-born neurons
(2019–2022) NHMRC Project Grant
Brain connectome: from synapse, large-scale network to behaviour
(2018–2020) ARC Discovery Projects
A specialised surgical and behavioural facility for longitudinal, multimodal examination of the rodent brain
(2018) UQ Major Equipment and Infrastructure
Insights into the encoding of memories through the circuitry of fear
Kenna, Matthew, Marek, Roger and Sah, Pankaj (2023). Insights into the encoding of memories through the circuitry of fear. Current Opinion in Neurobiology, 80 102712, 102712. doi: 10.1016/j.conb.2023.102712
The PPN and motor control: Preclinical studies to deep brain stimulation for Parkinson’s disease
Lin, Caixia, Ridder, Margreet C. and Sah, Pankaj (2023). The PPN and motor control: Preclinical studies to deep brain stimulation for Parkinson’s disease. Frontiers in Neural Circuits, 17. doi: 10.3389/fncir.2023.1095441
Optogenetic stimulation and spatial localization of neurons using a multi-OLED approach
Kielar, Marcin, Marek, Roger, Kenna, Matthew, Cole, Cameron M., Xu, Li, Yambem, Soniya D., Sah, Pankaj and Pandey, Ajay K. (2022). Optogenetic stimulation and spatial localization of neurons using a multi-OLED approach. ACS Photonics, 9 (10), 3279-3290. doi: 10.1021/acsphotonics.2c00590
Theta coupling within the medial prefrontal cortex regulates fear extinction and renewal
Wang, Cong, Stratton, Peter G., Sah, Pankaj and Marek, Roger (2022). Theta coupling within the medial prefrontal cortex regulates fear extinction and renewal. iScience, 25 (10) 105036, 1-14. doi: 10.1016/j.isci.2022.105036
Bhat, Gurudutt, Kielar, Marcin, Rao, Haixia, Gholami, Mahnaz D., Mathers, Isabel, Larin, Astrid C. R., Flanagan, Thomas, Erdenebileg, Enkhtur, Bruno, Annalisa, Pannu, Amandeep Singh, Fairfull‐Smith, Kathryn E., Izake, Emad L., Sah, Pankaj, Lam, Yeng Ming, Pandey, Ajay K. and Sonar, Prashant (2022). Versatile aza‐BODIPY ‐based low‐bandgap conjugated small molecule for light harvesting and near‐infrared photodetection. InfoMat, 4 (12) e12345. doi: 10.1002/inf2.12345
A midbrain-thalamus-cortex circuit reorganizes cortical dynamics to initiate movement
Inagaki, Hidehiko K., Chen, Susu, Ridder, Margreet C., Sah, Pankaj, Li, Nuo, Yang, Zidan, Hasanbegovic, Hana, Gao, Zhenyu, Gerfen, Charles R. and Svoboda, Karel (2022). A midbrain-thalamus-cortex circuit reorganizes cortical dynamics to initiate movement. Cell, 185 (6), 1065-1081. doi: 10.1016/j.cell.2022.02.006
Perumal, Madhusoothanan B. and Sah, Pankaj (2022). A protocol to investigate cellular and circuit mechanisms generating sharp wave ripple oscillations in rodent basolateral amygdala using ex vivo slices. STAR Protocols, 3 (1) 101085, 101085. doi: 10.1016/j.xpro.2021.101085
Hippocampus-prefrontal coupling regulates recognition memory for novelty discrimination
Wang, Cong, Furlong, Teri M., Stratton, Peter G., Lee, Conrad C. Y., Xu, Li, Merlin, Sam, Nolan, Christoph, Arabzadeh, Ehsan, Marek, Roger and Sah, Pankaj (2021). Hippocampus-prefrontal coupling regulates recognition memory for novelty discrimination. The Journal of Neuroscience, 41 (46), 9617-9632. doi: 10.1523/jneurosci.1202-21.2021
Bhat, Gurudutt, Liu, Qian, Kielar, Marcin, Hamada, Yuya, Michinobu, Tsuyoshi, Sah, Pankaj, Ko Kyaw, Aung Ko, Pandey, Ajay K. and Sonar, Prashant (2021). Energy-level manipulation in novel indacenodithiophene-based donor–acceptor polymers for near-infrared organic photodetectors. ACS Applied Materials and Interfaces, 13 (25) acsami.1c03643, 29866-29875. doi: 10.1021/acsami.1c03643
Saturated free fatty acids and association with memory formation
Wallis, Tristan P., Venkatesh, Bharat G., Narayana, Vinod K., Kvaskoff, David, Ho, Alan, Sullivan, Robert K., Windels, François, Sah, Pankaj and Meunier, Frédéric A. (2021). Saturated free fatty acids and association with memory formation. Nature Communications, 12 (1) 3443, 3443. doi: 10.1038/s41467-021-23840-3
Blackmore, Daniel G., Turpin, Fabrice, Palliyaguru, Tishila, Evans, Harrison T., Chicoteau, Antony, Lee, Wendy, Pelekanos, Matthew, Nguyen, Nghia, Song, Jae, Sullivan, Robert K. P., Sah, Pankaj, Bartlett, Perry F. and Götz, Jürgen (2021). Low-intensity ultrasound restores long-term potentiation and memory in senescent mice through pleiotropic mechanisms including NMDAR signaling. Molecular Psychiatry, 26 (11), 6975-6991. doi: 10.1038/s41380-021-01129-7
Harley, Samuel B. R., Willis, Emily F., Shaikh, Samreen N., Blackmore, Daniel G., Sah, Pankaj, Ruitenberg, Marc J., Bartlett, Perry F. and Vukovic, Jana (2021). Selective ablation of BDNF from microglia reveals novel roles in self-renewal and hippocampal neurogenesis. The Journal of Neuroscience, 41 (19), 4172-4186. doi: 10.1523/jneurosci.2539-20.2021
Perumal, Madhusoothanan B., Latimer, Benjamin, Xu, Li, Stratton, Peter, Nair, Satish and Sah, Pankaj (2021). Microcircuit mechanisms for the generation of sharp-wave ripples in the basolateral amygdala: A role for chandelier interneurons. Cell Reports, 35 (6) 109106, 1-16. doi: 10.1016/j.celrep.2021.109106
Inhibitory circuits in the basolateral amygdala in aversive learning and memory
Perumal, Madhusoothanan B. and Sah, Pankaj (2021). Inhibitory circuits in the basolateral amygdala in aversive learning and memory. Frontiers in Neural Circuits, 15, 633235. doi: 10.3389/fncir.2021.633235
Mosley, Philip E., Windels, François, Morris, John, Coyne, Terry, Marsh, Rodney, Giorni, Andrea, Mohan, Adith, Sachdev, Perminder, O’Leary, Emily, Boschen, Mark, Sah, Pankaj and Silburn, Peter A. (2021). A randomised, double-blind, sham-controlled trial of deep brain stimulation of the bed nucleus of the stria terminalis for treatment-resistant obsessive-compulsive disorder. Translational Psychiatry, 11 (1) 190, 190. doi: 10.1038/s41398-021-01307-9
Direct detection of neuronal activity using organic photodetectors
Kielar, Marcin, Gooch, Helen, Xu, Li, Pandey, Ajay K. and Sah, Pankaj (2020). Direct detection of neuronal activity using organic photodetectors. ACS Photonics, 8 (1) acsphotonics.0c01378, 228-237. doi: 10.1021/acsphotonics.0c01378
Sun, Yajie, Qian, Lei, Xu, Li, Hunt, Sarah and Sah, Pankaj (2020). Somatostatin neurons in the central amygdala mediate anxiety by disinhibition of the central sublenticular extended amygdala. Molecular Psychiatry. doi: 10.1038/s41380-020-00894-1
Editorial: Integrative Brain Function Down Under
Sah, Pankaj, Stuart, Greg J. and Egan, Gary F. (2020). Editorial: Integrative Brain Function Down Under. Frontiers in Neural Circuits, 14 48, 48. doi: 10.3389/fncir.2020.00048
Diversity of interneurons in the lateral and basal amygdala
Polepalli, Jai S., Gooch, Helen and Sah, Pankaj (2020). Diversity of interneurons in the lateral and basal amygdala. npj Science of Learning, 5 (1) 10, 10. doi: 10.1038/s41539-020-0071-z
Fear conditioning and the basolateral amygdala [version 1; peer review: 3 approved]
Sun, Yajie, Gooch, Helen and Sah, Pankaj (2020). Fear conditioning and the basolateral amygdala [version 1; peer review: 3 approved]. F1000Research, 9 53. doi: 10.12688/f1000research.21201.1
Light detection in open-circuit voltage mode of organic photodetectors
Kielar, Marcin, Hamid, Tasnuva, Wiemer, Martin, Windels, François, Hirsch, Lionel, Sah, Pankaj and Pandey, Ajay K. (2019). Light detection in open-circuit voltage mode of organic photodetectors. Advanced Functional Materials, 30 (9) 1907964, 1-9. doi: 10.1002/adfm.201907964
Gooch, Helen, Cui, Xiaoying, Anggono, Victor, Trzaskowski, Maciej, Tan, Men Chee, Eyles, Darryl W., Burne, Thomas H. J., Jang, Se Eun, Mattheisen, Manuel, Hougaard, David M., Pedersen, Bent Nørgaard, Cohen, Arieh, Mortensen, Preben B., Sah, Pankaj and McGrath, John J. (2019). 1,25-Dihydroxyvitamin D modulates L-type voltage-gated calcium channels in a subset of neurons in the developing mouse prefrontal cortex. Translational Psychiatry, 9 (1) 281, 281. doi: 10.1038/s41398-019-0626-z
Interneurons in the prefrontal cortex: a role in the genesis of anxiety in adolescence?
Sah, Pankaj (2019). Interneurons in the prefrontal cortex: a role in the genesis of anxiety in adolescence?. Biological Psychiatry, 86 (9), 650-651. doi: 10.1016/j.biopsych.2019.07.026
Spectral changes associated with transmission of OLED emission through human skin
Yambem, Soniya D., Brooks-Richards, Trent L., Forrestal, David P., Kielar, Marcin, Sah, Pankaj, Pandey, Ajay K. and Woodruff, Maria A. (2019). Spectral changes associated with transmission of OLED emission through human skin. Scientific Reports, 9 (1) 9875, 9875. doi: 10.1038/s41598-019-45867-9
Organic optoelectronic diodes as tactile sensors for soft-touch applications
Kielar, Marcin, Hamid, Tasnuva, Wu, Liao, Windels, François, Sah, Pankaj and Pandey, Ajay K. (2019). Organic optoelectronic diodes as tactile sensors for soft-touch applications. ACS Applied Materials & Interfaces, 11 (24) acsami.9b04671, 21775-21783. doi: 10.1021/acsami.9b04671
rSK1 in rat neurons: a controller of membrane rSK2?
Autuori, Eleonora, Sedlak, Petra, Xu, Li, C. Ridder, Margreet, Tedoldi, Angelo and Sah, Pankaj (2019). rSK1 in rat neurons: a controller of membrane rSK2?. Frontiers in Neural Circuits, 13 21, 21. doi: 10.3389/fncir.2019.00021
Neural circuits for a top-down control of fear and extinction
Marek, Roger, Sun, Yajie and Sah, Pankaj (2019). Neural circuits for a top-down control of fear and extinction. Psychopharmacology, 236 (1), 313-320. doi: 10.1007/s00213-018-5033-2
Elongator mutation in mice induces neurodegeneration and ataxia-like behavior
Kojic, Marija, Gaik, Monika, Kiska, Bence, Salerno-Kochan, Anna, Hunt, Sarah, Tedoldi, Angelo, Mureev, Sergey, Jones, Alun, Whittle, Belinda, Genovesi, Laura A., Adolphe, Christelle, Brown, Darren L., Stow, Jennifer L., Alexandrov, Kirill, Sah, Pankaj, Glatt, Sebastian and Wainwright, Brandon J. (2018). Elongator mutation in mice induces neurodegeneration and ataxia-like behavior. Nature Communications, 9 (1) 3195, 3195. doi: 10.1038/s41467-018-05765-6
The association between neonatal vitamin D status and risk of schizophrenia
Eyles, Darryl W., Trzaskowski, Maciej, Vinkhuyzen, Anna A. E., Mattheisen, Manuel, Meier, Sandra, Gooch, Helen, Anggono, Victor, Cui, Xiaoying, Tan, Men Chee, Burne, Thomas H. J., Jang, Se Eun, Kvaskoff, David, Hougaard, David M., Nørgaard-Pedersen, Bent, Cohen, Arieh, Agerbo, Esben, Pedersen, Carsten B., Børglum, Anders D., Mors, Ole, Sah, Pankaj, Wray, Naomi R., Mortensen, Preben B. and McGrath, John J. (2018). The association between neonatal vitamin D status and risk of schizophrenia. Scientific Reports, 8 (1) 17692, 17692. doi: 10.1038/s41598-018-35418-z
Marek, Roger, Jin, Jingji, Goode, Travis D., Giustino, Thomas F., Wang, Qian, Acca, Gillian M., Holehonnur, Roopashri, Ploski, Jonathan E., Fitzgerald, Paul J., Lynagh, Timothy, Lynch, Joseph W., Maren, Stephen and Sah, Pankaj (2018). Author Correction: Hippocampus-driven feed-forward inhibition of the prefrontal cortex mediates relapse of extinguished fear. Nature Neuroscience, 21 (9), 1291-1291. doi: 10.1038/s41593-018-0183-4
Marek, Roger, Xu, Li, Sullivan, Robert K. P. and Sah, Pankaj (2018). Excitatory connections between the prelimbic and infralimbic medial prefrontal cortex show a role for the prelimbic cortex in fear extinction. Nature Neuroscience, 21 (5), 654-658. doi: 10.1038/s41593-018-0137-x
GABA A receptors and inhibitory neurotransmission in the amygdalar complex
Perumal, Madhusoothanan B., Lynch, Joseph W. and Sah, Pankaj (2018). GABA A receptors and inhibitory neurotransmission in the amygdalar complex. Current Opinion in Physiology, 2, 58-64. doi: 10.1016/j.cophys.2018.01.003
The nature and nurture of education
Sah, Pankaj, Fanselow, Michael, Quirk, Gregory J., Hattie, John, Mattingley, Jason and Tokuhama-Espinosa, Tracey (2018). The nature and nurture of education. npj Science of Learning, 3 (1) 6, 6. doi: 10.1038/s41539-018-0023-z
Marek, Roger, Jin, Jingji, Goode, Travis D., Giustino, Thomas F., Wang, Qian, Acca, Gillian M., Holehonnur, Roopashri, Ploski, Jonathan E., Fitzgerald, Paul J., Lynagh, Timothy, Lynch, Joseph W., Maren, Stephen and Sah, Pankaj (2018). Hippocampus-driven feed-forward inhibition of the prefrontal cortex mediates relapse of extinguished fear. Nature Neuroscience, 21 (3), 1-9. doi: 10.1038/s41593-018-0073-9
Blackmore, Daniel G., Turpin, Fabrice, Mohamed, Abdalla Z., Zong, Fangrong, Pandit, Rucha, Pelekanos, Matthew, Nasrallah, Fatima, Sah, Pankaj, Bartlett, Perry F. and Gotz, Juergen (2018). Multimodal analysis of aged wild-type mice exposed to repeated scanning ultrasound treatments demonstrates long-term safety. Theranostics, 8 (22), 6233-6247. doi: 10.7150/thno.27941
Giorni, Andrea, Windels, François, Stratton, Peter G., Cook, Raymond, Silberstein, Paul, Coyne, Terrence, Silburn, Peter A. and Sah, Pankaj (2017). Single-unit activity of the anterior Globus pallidus internus in Tourette patients and posterior Globus pallidus internus in dystonic patients. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology, 128 (12), 2510-2518. doi: 10.1016/j.clinph.2017.10.003
Fear, anxiety, and the amygdala
Sah, P. (2017). Fear, anxiety, and the amygdala. Neuron, 96 (1), 1-2. doi: 10.1016/j.neuron.2017.09.013
Evidence for newly generated interneurons in the basolateral amygdala of adult mice
Jhaveri, D. J., Tedoldi, A., Hunt, S., Sullivan, R., Watts, N. R., Power, J. M., Bartlett, P. F. and Sah, P. (2017). Evidence for newly generated interneurons in the basolateral amygdala of adult mice. Molecular Psychiatry, 23 (3), 521-532. doi: 10.1038/mp.2017.134
May, Linda M., Anggono, Victor, Gooch, Helen M., Jang, Se E., Matusica, Dusan, Kerbler, Georg M., Meunier, Frederic A., Sah, Pankaj and Coulson, Elizabeth J. (2017). G-protein-coupled inwardly rectifying potassium (GIRK) channel activation by the p75 neurotrophin receptor is required for amyloid beta toxicity. Frontiers in Neuroscience, 11 (455) 455. doi: 10.3389/fnins.2017.00455
Calcium signalling in medial intercalated cell dendrites and spines
Strobel, Cornelia, Sullivan, Robert K. P., Stratton, Peter and Sah, Pankaj (2017). Calcium signalling in medial intercalated cell dendrites and spines. The Journal of Physiology, 595 (16), 5653-5669. doi: 10.1113/JP274261
Dixon, Christine L., Sah, Pankaj, Keramidas, Angelo, Lynch, Joseph W. and Durisic, Nela (2017). Gamma 1-containing GABA-A receptors cluster at synapses where they mediate slower synaptic currents than gamma 2-containing GABA-A receptors. Frontiers in Molecular Neuroscience, 10 178, 178. doi: 10.3389/fnmol.2017.00178
Islam, Robiul, Zhang, Yan, Xu, Li, Sah, Pankaj and Lynch, Joseph W. (2017). A chemogenetic receptor that enhances the magnitude and frequency of glycinergic inhibitory postsynaptic currents without inducing a tonic chloride flux. ACS Chemical Neuroscience, 8 (3), 460-467. doi: 10.1021/acschemneuro.6b00382
Intrinsic circuits in the lateral central amygdala
Hunt, Sarah, Sun, Yajie, Kucukdereli, Hakan, Klein, Ruediger and Sah, Pankaj (2017). Intrinsic circuits in the lateral central amygdala. Eneuro, 4 (1) e0367-16.2017, ENEURO.0367-16.2017. doi: 10.1523/ENEURO.0367-16.2017
Islam, Robiul, Keramidas, Angelo, Xu, Li, Durisic, Nela, Sah, Pankaj and Lynch, Joseph W. (2016). Ivermectin-activated, cation-permeable glycine receptors for the chemogenetic control of neuronal excitation. ACS Chemical Neuroscience, 7 (12), 1647-1657. doi: 10.1021/acschemneuro.6b00168
Neuroscience and education: mind the gap
Morris, John and Sah, Pankaj (2016). Neuroscience and education: mind the gap. Australian Journal of Education, 60 (2), 146-156. doi: 10.1177/0004944116652913
Windels, Francois, Yan, Shanzhi, Stratton, Peter G., Sullivan, Robert, Crane, James W. and Sah, Pankaj (2016). Auditory tones and foot-shock recapitulate spontaneous sub-threshold activity in basolateral amygdala principal neurons and interneurons. PLoS ONE, 11 (5) e0155192, e0155192. doi: 10.1371/journal.pone.0155192
Integrating neuroscience and learning: now’s the time
Sah, Pankaj, Fanselow, Michael, Hattie, John, Magsamen, Susan, Mattingley, Jason, Quirk, Gregory and Williams, Stephen (2016). Integrating neuroscience and learning: now’s the time. n p j Science of Learning, 1 (1) 16007, 16007. doi: 10.1038/npjscilearn.2016.7
IK1 channels do not contribute to the slow after hyperpolarization in pyramidal neurons
Wang, Kang, Mateos-Aparicio, Pedro, Honigsperger, Christoph, Raghuram, Vijeta, Wu, Wendy W., Ridder, Margreet C., Sah, Pankaj, Mayile, Jim, Storm, Johan F. and Adelman, John P. (2016). IK1 channels do not contribute to the slow after hyperpolarization in pyramidal neurons. eLife, 5 (e11206) e11206, 1-16. doi: 10.7554/eLife.11206
Spampanato, Jay, Sullivan, Robert K. P., Perumal, Madhusoothanan B. and Sah, Pankaj (2016). Development and physiology of GABAergic feedback excitation in parvalbumin expressing interneurons of the mouse basolateral amygdala. Physiological Reports, 4 (e12664) e12664, 1-15. doi: 10.14814/phy2.12664
Corrigendum: Regulating anxiety with extrasynaptic inhibition
Botta, Paolo, Demmou, Lynda, Kasugai, Yu, Markovic, Milica, Xu, Chun, Fadok, Jonathan P, Lu, Tingjia, Poe, Michael M, Xu, Li, Cook, James M, Rudolph, Uwe, Sah, Pankaj, Ferraguti, Francesco and Lüthi, Andreas (2015). Corrigendum: Regulating anxiety with extrasynaptic inhibition. Nature Neuroscience, 18 (12), 1862-1862. doi: 10.1038/nn1215-1862a
Regulating anxiety with extrasynaptic inhibition
Botta, Paolo, Demmou, Lynda, Kasugai, Yu, Markovic, Milica, Xu, Chun, Fadok, Jonathan P., Lu, Tingjia, Poe, Michael M., Xu, Li, Cook, James M., Rudolph, Uwe, Sah, Pankaj, Ferraguti, Francesco and Luthi, Andreas (2015). Regulating anxiety with extrasynaptic inhibition. Nature Neuroscience, 18 (10), 1493-1500. doi: 10.1038/nn.4102
Dendritic organization of olfactory inputs to medial amygdala neurons
Keshavarzi, Sepideh, Power, John M., Albers, Eva H.H., Sullivan, Robert K.S. and Sah, Pankaj (2015). Dendritic organization of olfactory inputs to medial amygdala neurons. Journal of Neuroscience, 35 (38), 13020-13028. doi: 10.1523/JNEUROSCI.0627-15.2015
Sun, Yajie, Hunt, Sarah and Sah, Pankaj (2015). Norepinephrine and Corticotropin-Releasing Hormone: Partners in the Neural Circuits that Underpin Stress and Anxiety. Neuron, 87 (3), 468-470. doi: 10.1016/j.neuron.2015.07.022
Where and what is the PPN and what is its role in locomotion?
Windels, Francois, Thevathasan, Wesley, Silburn, Peter and Sah, Pankaj (2015). Where and what is the PPN and what is its role in locomotion?. Brain, 138 (5), 1133-1134. doi: 10.1093/brain/awv059
Vitamin D and the brain: key questions for future research
Cui, Xiaoying, Gooch, Helen, Groves, Natalie J., Sah, Pankaj, Burne, Thomas H., Eyles, Darryl W. and McGrath, John J. (2015). Vitamin D and the brain: key questions for future research. The Journal of Steroid Biochemistry and Molecular Biology, 148, 305-309. doi: 10.1016/j.jsbmb.2014.11.004
Prefrontal and auditory input to intercalated neurons of the Amygdala
Strobel, Cornelia, Marek, Roger, Gooch, Helen, Sullivan, Robert K. P. and Sah, Pankaj (2015). Prefrontal and auditory input to intercalated neurons of the Amygdala. Cell Reports, 10 (9), 1435-1442. doi: 10.1016/j.celrep.2015.02.008
Widagdo, Jocelyn, Chai, Ye Jin, Ridder, Margreet C, Chau, Yu Qian, Johnson, Richard C, Sah, Pankaj, Huganir, Richard L and Anggono, Victor (2015). Activity-Dependent ubiquitination of GluA1 and GluA2 regulates AMPA receptor intracellular sorting and degradation. Cell Reports, 10 (5), 783-795. doi: 10.1016/j.celrep.2015.01.015
Dendritic spine heterogeneity and calcium dynamics in basolateral amygdala principal neurons
Power, John M. and Sah, Pankaj P. (2014). Dendritic spine heterogeneity and calcium dynamics in basolateral amygdala principal neurons. Journal of Neurophysiology, 112 (7), 1616-1627. doi: 10.1152/jn.00770.2013
Balanced interhemispheric cortical activity is required for correct targeting of the corpus callosum
Suarez, Rodrigo, Fenlon, Laura R., Marek, Roger, Avitan, Lilach A, Sah, Pankaj, Goodhill, Geoffrey J. and Richards, Linda J. (2014). Balanced interhemispheric cortical activity is required for correct targeting of the corpus callosum. Neuron, 82 (6), 1289-1298. doi: 10.1016/j.neuron.2014.04.040
Emotional regulation of pain: the role of noradrenaline in the amygdala
Strobel, Cornelia, Hunt, Sarah, Sullivan, Robert, Sun, Jian and Sah, Pankaj (2014). Emotional regulation of pain: the role of noradrenaline in the amygdala. Science China Life Sciences, 57 (4), 384-390. doi: 10.1007/s11427-014-4638-x
GABAa receptor α and γ subunits shape synaptic currents via different mechanisms
Dixon, Christine, Sah, Pankaj, Lynch, Joseph W. and Keramidas, Angelo (2014). GABAa receptor α and γ subunits shape synaptic currents via different mechanisms. Journal of Biological Chemistry, 289 (9), 5399-5411. doi: 10.1074/jbc.M113.514695
Rodent scope: A user-configurable digital wireless telemetry system for freely behaving animals
Ball, David, Kliese, Russell, Windels, Francois, Nolan, Christopher, Stratton, Peter, Sah, Panjkaj and Wiles, Janet (2014). Rodent scope: A user-configurable digital wireless telemetry system for freely behaving animals. PLoS One, 9 (2) e89949, e89949.1-e89949.10. doi: 10.1371/journal.pone.0089949
Mechanisms of heterosynaptic metaplasticity
Hulme, Sarah R., Jones, Owen D., Raymond, Clarke R., Sah, Pankaj and Abraham, Wickliffe C. (2014). Mechanisms of heterosynaptic metaplasticity. Philosophical Transactions of the Royal Society B: Biological Sciences, 369 (1633) 20130148, 1633.1-1633.8. doi: 10.1098/rstb.2013.0148
Functional properties and projections of neurons in the medial amygdala
Keshavarzi, Sepideh, Sullivan, Robert K. P., Ianno, Damian J. and Sah, Pankaj (2014). Functional properties and projections of neurons in the medial amygdala. Journal of Neuroscience, 34 (26), 8699-8715. doi: 10.1523/JNEUROSCI.1176-14.2014
Imagined gait modulates neuronal network dynamics in the human pedunculopontine nucleus
Tattersall, Timothy L., Stratton, Peter G., Coyne, Terry J., Cook, Raymond, Silberstein, Paul, Silburn, Peter A., Windels, Francois and Sah, Pankaj (2014). Imagined gait modulates neuronal network dynamics in the human pedunculopontine nucleus. Nature Neuroscience, 17 (3), 449-454. doi: 10.1038/nn.3642
The amygdala and medial prefrontal cortex: partners in the fear circuit
Marek, Roger, Strobel, Cornelia, Bredy, Timothy W. and Sah, Pankaj (2013). The amygdala and medial prefrontal cortex: partners in the fear circuit. Journal of Physiology, 591 (10), 2381-2391. doi: 10.1113/jphysiol.2012.248575
Delaney, Andrew J., Sedlak, Petra L., Autuori, Eleonora, Power, John M. and Sah, Pankaj (2013). Synaptic NMDA receptors in basolateral amygdala principal neurons are triheteromeric proteins: physiological role of GluN2B subunits. Journal of Neurophysiology, 109 (5), 1391-1402. doi: 10.1152/jn.00176.2012
Lesions of the basal forebrain cholinergic system in mice disrupt idiothetic navigation
Hamlin, Adam S., Windels, Francois, Boskovic, Zoran, Sah, Pankaj and Coulson, Elizabeth J. (2013). Lesions of the basal forebrain cholinergic system in mice disrupt idiothetic navigation. PLoS ONE, 8 (1) e53472, e53472.1-e53472.9. doi: 10.1371/journal.pone.0053472
Properties of doublecortin expressing neurons in the adult mouse dentate gyrus
Spampanato, Jay, Sullivan, Robert K., Turpin, Fabrice R., Bartlett, Perry F. and Sah, Pankaj (2012). Properties of doublecortin expressing neurons in the adult mouse dentate gyrus. PLoS One, 7 (9) e41029, e41029-1-e41029-12. doi: 10.1371/journal.pone.0041029
p300/CBP-associated factor selectively regulates the extinction of conditioned fear
Wei, Wei, Coelho, Carlos M., Li, Xiang, Marek, Roger, Yan, Shanzhi, Anderson, Shawn, Meyers, David, Mukherjee, Chandrani, Sbardella, Gianluca, Castellano, Sabrina, Milite, Ciro, Rotili, Dante, Mai, Antonello, Cole, Philip A., Sah, Pankaj, Kobor, Michael S. and Bredy, Timothy W. (2012). p300/CBP-associated factor selectively regulates the extinction of conditioned fear. Journal of Neuroscience, 32 (35), 11930-11941. doi: 10.1523/JNEUROSCI.0178-12.2012
Targeted ablation of oligodendrocytes induces axonal pathology independent of overt demyelination
Oluich, Laura-Jane, Stratton, Jo Anne S., Xing, Yao Lulu, Ng, Sze Woei, Cate, Holly S., Sah, Pankaj, Windels, Francois, Kilpatrick, Trevor J. and Merson, Tobias D. (2012). Targeted ablation of oligodendrocytes induces axonal pathology independent of overt demyelination. The Journal of Neuroscience, 32 (24), 8317-8330. doi: 10.1523/JNEUROSCI.1053-12.2012
Action potential waveform variability limits multi-unit separation in freely behaving rats
Stratton, Peter, Cheung, Allen, Wiles, Janet H., Kiyatkin, E., Sah, Pankaj and Windels, Francois (2012). Action potential waveform variability limits multi-unit separation in freely behaving rats. PLoS One, 7 (6) e38482, e38482.1-e38482.16. doi: 10.1371/journal.pone.0038482
An introduction to a symposium dedicated to the scientific achievements of Roger Nicoll
Jahr, Craig, Perkel, David and Sah, Pankaj (2012). An introduction to a symposium dedicated to the scientific achievements of Roger Nicoll. Journal of Physiology, 590 (10), 2201-2202. doi: 10.1113/jphysiol.2012.230896
Delaney, Andrew J., Power, John M. and Sah, Pankaj (2012). Ifenprodil reduces excitatory synaptic transmission by blocking presynaptic P/Q type calcium channels. Journal of Neurophysiology, 107 (6), 1571-1575. doi: 10.1152/jn.01066.2011
Small-conductance Ca(2+)-activated K(+) channels: form and function
Adelman, John P., Maylie, James and Sah, Pankaj (2012). Small-conductance Ca(2+)-activated K(+) channels: form and function. Annual Review of Physiology, 74, 245-269. doi: 10.1146/annurev-physiol-020911-153336
Marek, Roger, Coelho, Carlos M., Sullivan, Robert K. P., Baker-Andresen, Danay, Li, Xiang, Ratnu, Vikram, Dudley, Kevin J., Meyers, David, Mukherjee, Chandrani, Cole, Philip A., Sah, Pankaj and Bredy, Timothy W. (2011). Paradoxical enhancement of fear extinction memory and synaptic plasticity by inhibition of the histone acetyltransferase p300. Journal of Neuroscience, 31 (20), 7486-7491. doi: 10.1523/JNEUROSCI.0133-11.2011
Interneurons in the basolateral amygdala
Spampanato, Jay, Polepalli, Jai and Sah, Pankaj (2011). Interneurons in the basolateral amygdala. Neuropharmacology, 60 (5), 765-773. doi: 10.1016/j.neuropharm.2010.11.006
Location and function of the slow afterhyperpolarization channels in the basolateral amygdala
Power, John M., Bocklisch, Christina, Curby, Peter and Sah, Pankaj (2011). Location and function of the slow afterhyperpolarization channels in the basolateral amygdala. Journal of Neuroscience, 31 (2), 526-537. doi: 10.1523/JNEUROSCI.1045-10.2011
Inhibition dominates the early phase of up-states in the basolateral amygdala
Windels, Francois, Crane, James W. and Sah, Pankaj (2010). Inhibition dominates the early phase of up-states in the basolateral amygdala. Journal of Neurophysiology, 104 (6), 3433-3438. doi: 10.1152/jn.00531.2010
A Specific class of interneuron mediates inhibitory plasticity in the lateral amygdala
Polepalli, Jai S., Sullivan, Robert K. P., Yanagawa, Yuchio and Sah, Pankaj (2010). A Specific class of interneuron mediates inhibitory plasticity in the lateral amygdala. Journal of Neuroscience, 30 (44), 14619-14629. doi: 10.1523/JNEUROSCI.3252-10.2010
Differential expression of glycine receptor subunits in the rat basolateral and central amygdala
Delaney, A. J., Esmaeili, A., Sedlak, Petra L., Lynch, J. W. and Sah, P. (2010). Differential expression of glycine receptor subunits in the rat basolateral and central amygdala. Neuroscience Letters, 469 (2), 237-242. doi: 10.1016/j.neulet.2009.12.003
Crane, James W., Windels, Francois and Sah, Pankaj (2009). Oscillations in the basolateral amygdala: aversive stimulation is state dependent and resets the oscillatory phase. Journal Neurophysiology, 102 (3), 1379-1387. doi: 10.1152/jn.00438.2009
Esmaeili, Abolghasem, Lynch, Joseph W. and Sah, Pankaj (2009). GABAᴀ receptors containing gamma 1 subunits contribute to inhibitory transmission in the central amygdala. Journal of Neurophysiology, 101 (1), 341-349. doi: 10.1152/jn.90991.2008
Crane, James W., Baiquni, Gilang P., Sullivan, Robert K. P., Lee, John D, Sah, Pankaj, Taylor, Stephen M, Noakes, Peter G. and Woodruff, Trent (2009). The C5a anaphylatoxin receptor CD88 is expressed in presynaptic terminals of hippocampal mossy fibres. Journal of Neuroinflammation, 6 (34) 34, 34.1-34.10. doi: 10.1186/1742-2094-6-34
Faber, Elizabeth S. L., Delaney, Andrew J., Power, John M., Sedlak, Petra, Crane, James W. and Sah, Pankaj (2008). Modulation of SK Channel Trafficking by Beta Adrenoceptors Enhances Excitatory Synaptic Transmission and Plasticity in the Amygdala. Journal of Neuroscience, 28 (43), 10803-10813. doi: 10.1523/JNEUROSCI.1796-08.2008
Behavioural neuroscience: The circuit of fear
Sah, Pankaj and Westbrook, R. Federick (2008). Behavioural neuroscience: The circuit of fear. Nature, 454 (7204), 589-590. doi: 10.1038/454589a
Fear Conditioning and Long-term Potentiation in the Amygdala What Really Is the Connection?
Sah, Pankaj, Westbrook, R. F. and Luthi, A. (2008). Fear Conditioning and Long-term Potentiation in the Amygdala What Really Is the Connection?. Annals of the New York Academy of Science, 1129, 88-95. doi: 10.1196/annals.1417.020
Latent stem and progenitor cells in the hippocampus are activated by neural excitation
Walker, Tara L., White, Amanda, Black, Debra M., Wallace, Robyn H., Sah, Pankaj and Bartlett, Perry F. (2008). Latent stem and progenitor cells in the hippocampus are activated by neural excitation. Journal of Neuroscience, 28 (20), 5240-5247. doi: 10.1523/JNEUROSCI.0344-08.2008
Power, John M. and Sah, Pankaj (2008). Competition between Calcium-Activated K⁺ Channels Determines Cholinergic Action on Firing Properties of Basolateral Amygdala Projection Neurons. Journal of Neuroscience, 28 (12), 3209-3220. doi: 10.1523/JNEUROSCI.4310-07.2008
Coulson, E. J., May, L. M., Osborne, S. L., Reid, K., Underwood, C. K., Meunier, F. A., Bartlett, P. F. and Sah, P. (2008). p75 neurotrophin receptor mediates neuronal cell death by activating GIRK channels through phosphatidylinositol 4,5-bisphosphate. Journal of Neuroscience, 28 (1), 315-324. doi: 10.1523/JNEUROSCI.2699-07.2008
Woodruff, Alan R. and Sah, Pankaj (2007). Inhibition and synchronization of basal amygdala principal neuron spiking by parvalbumin-positive interneurons. Journal of Neurophysiology, 98 (5), 2956-2961. doi: 10.1152/jn.00739.2007
Power, John M. and Sah, Pankaj (2007). Distribution of IP3-mediated calcium responses and their role in nuclear signalling in rat basolateral amygdala neurons. Journal of Physiology, 580 (3), 835-857. doi: 10.1113/jphysiol.2006.125062
Bidirectional synaptic plasticity at nociceptive afferents in the rat central amygdala
Lopez de Armentia, M. and Sah, P. (2007). Bidirectional synaptic plasticity at nociceptive afferents in the rat central amygdala. Journal of Physiology, 581 (3), 961-970. doi: 10.1113/jphysiol.2007.101822
Functions of SK channels in central neurons
Faber, E. S. L. and Sah, P. (2007). Functions of SK channels in central neurons. Clinical and Experimental Pharmacology and Physiology, 34 (10), 1077-1083. doi: 10.1111/j.1440-1681.2007.04725.x
Networks of parvalbumin-positive interneurons in the basolateral amygdala
Woodruff, Alan R. and Sah, Pankaj (2007). Networks of parvalbumin-positive interneurons in the basolateral amygdala. Journal of Neuroscience, 27 (3), 553-563. doi: 10.1523/JNEUROSCI.3686-06.2007
Noradrenaline modulates transmission at a central synapse by a presynaptic mechanism
Delaney, Andrew J., Crane, James W. and Sah, Pankaj (2007). Noradrenaline modulates transmission at a central synapse by a presynaptic mechanism. Neuron, 56 (5), 880-892. doi: 10.1016/j.neuron.2007.10.022
Sez-6 proteins affect dendritic arborization patterns and excitability of cortical pyramidal neurons
Gunnersen, J. M., Kim, M. H., Fuller, S. J., De Silva, M., Britto, J. M., Hammond, V. E., Davies, P. J., Petrou, S., Faber, E. S. L., Sah, P. and Tan, S. S. (2007). Sez-6 proteins affect dendritic arborization patterns and excitability of cortical pyramidal neurons. Neuron, 56 (4), 621-639. doi: 10.1016/j.neuron.2007.09.018
Sah, Pankaj (2006). Canonical organization of opioid modulation of nociceptive circuits. Focus on "mu opioid receptor activation inhibits GABAergic inputs to basolateral amygdala neurons through Kv1.1/Kv1.2 channels". Journal of Neurophysiology, 95 (4), 2029-2030. doi: 10.1152/jn.01259.2005
Graham, B. A., Schofield, P. R., Sah, P., Margrie, T. W. and Callister, R. J. (2006). Distinct physiological mechanisms underlie altered glycinergic synaptic transmission in the murine mutants spastic, spasmodic, and oscillator. Journal of Neuroscience, 26 (18), 4880-4890. doi: 10.1523/JNEUROSCI.3991-05.2006
GABAergic excitation in the basolateral amygdala
Woodruff, Alan R., Monyer, Hannah and Sah, Pankaj (2006). GABAergic excitation in the basolateral amygdala. Journal of Neuroscience, 26 (46), 11881-11887. doi: 10.1523/JNEUROSCI.3389-06.2006
Faber, E. S. L., Sedlak, P., Vidovic, M. and Sah, P. (2006). Synaptic activation of transient receptor potential channels by metabotropic glutamate receptors in the lateral amygdala. Neuroscience, 137 (3), 781-794. doi: 10.1016/j.neuroscience.2005.09.027
Faber, E. S. L. and Sah, P. (2005). Independent roles of calcium and voltage-dependent potassium currents in controlling spike frequency adaptation in lateral amygdala pyramidal neurons. European Journal of Neuroscience, 22 (7), 1627-1635. doi: 10.1111/j.1460-9568.2005.04357.x
Power, J. M. and Sah, P. (2005). Intracellular calcium store filling by an L-type calcium current in the basolateral amygdala at subthreshold membrane potentials. Journal of Physiology, 562 (2), 439-453. doi: 10.1113/jphysiol.2004.076711
SK channels regulate excitatory synaptic transmission and plasticity in the lateral amygdala
Faber, E. S., Delaney, A. J. and Sah, P. (2005). SK channels regulate excitatory synaptic transmission and plasticity in the lateral amygdala. Nature Neuroscience, 8 (5), 635-641. doi: 10.1038/nn1450
Firing properties and connectivity of neurons in the rat lateral central nucleus of the amygdala
De Armentia, Mikel Lopez and Sah, Pankaj (2004). Firing properties and connectivity of neurons in the rat lateral central nucleus of the amygdala. Journal of Neurophysiology, 92 (3), 1285-1294. doi: 10.1152/jn.00211.2004
Opioids Inhibit Lateral Amygdala Pyramidal Neurons by Enhancing A Dendritic Potassium Current
Faber, E. S. L. and Sah, P. (2004). Opioids Inhibit Lateral Amygdala Pyramidal Neurons by Enhancing A Dendritic Potassium Current. Journal of Neuroscience, 24 (12), 3031-3039. doi: 10.1523/JNEUROSCI.4496-03.2004
Graham, BA, Schofield, PR, Sah, P and Callister, RJ (2003). Altered inhibitory synaptic transmission in superficial dorsal horn neurones in spastic and oscillator mice. Journal of Physiology-london, 551 (3), 905-916. doi: 10.1113/jphysiol.2003.049064
Faber, E. S. L. and Sah, P. (2003). Ca2+-activated K+(BK) channel inactivation contributes to spike broadening during repetitive firing in the rat lateral amygdala. Journal of Physiology, 552 (2), 483-497. doi: 10.1113/jphysiol.2003.050120
Calcium-activated potassium channels: Multiple contributions to neuronal function
Faber, ESL and Sah, P (2003). Calcium-activated potassium channels: Multiple contributions to neuronal function. Neuroscientist, 9 (3), 181-194. doi: 10.1177/107385840325673
De Armentia, Mikel Lopez and Sah, Pankaj (2003). Development and subunit composition of synaptic NMDA receptors in the amygdala: NR2B Synapses in the adult central amygdala. Journal of Neuroscience, 23 (17), 6876-6883. doi: 10.1523/jneurosci.23-17-06876.2003
Excitatory synaptic transmission in the lateral and central amygdala
Sah, P. and De Armentia, M. L. (2003). Excitatory synaptic transmission in the lateral and central amygdala. Annals of New York Academy of Sciences, 985 (THE AMYGDALA IN BRAIN FUNCTION Basic and Clinical Approaches), 67-77. doi: 10.1111/j.1749-6632.2003.tb07072.x
The amygdaloid complex: Anatomy and physiology
Sah, P, Faber, ESL, De Armentia, ML and Power, J (2003). The amygdaloid complex: Anatomy and physiology. Physiological Reviews, 83 (3), 803-834. doi: 10.1152/physrev.00002.2003
Channels underlying neuronal calcium-activated potassium currents
Sah, Pankaj and Faber, E S Louise (2002). Channels underlying neuronal calcium-activated potassium currents. Progress in Neurobiology, 66 (5), 345-53. doi: 10.1016/S0301-0082(02)00004-7
Channels underlying neuronal calcium-activated potassium currents
Sah, P and Faber, ESL (2002). Channels underlying neuronal calcium-activated potassium currents. Progress In Neurobiology, 66 (5), 345-353. doi: 10.1016/S0301-0082(02)00004-7
Neurobiology - Never fear, cannabinoids are here
Sah, P (2002). Neurobiology - Never fear, cannabinoids are here. Nature, 418 (6897), 488-489. doi: 10.1038/418488b
Nuclear calcium signaling evoked by cholinergic stimulation in hippocampal CA1 pyramidal neurons
Power, JM and Sah, P (2002). Nuclear calcium signaling evoked by cholinergic stimulation in hippocampal CA1 pyramidal neurons. Journal of Neuroscience, 22 (9), 3454-3462.
Physiological role of calcium-activated potassium currents in the rat lateral amygdala
Faber, ESL and Sah, P (2002). Physiological role of calcium-activated potassium currents in the rat lateral amygdala. Journal of Neuroscience, 22 (5), 1618-1628. doi: 10.1523/jneurosci.22-05-01618.2002
Faber, ESL, Callister, RJ and Sah, P (2001). Morphological and electrophysiological properties of principal neurons in the rat lateral amygdala in vitro. Journal of Neurophysiology, 85 (2), 714-723. doi: 10.1152/jn.2001.85.2.714
Delaney, A. J. and Sah, P. (2001). Pathway-specific targeting of GAB(A) a receptor subtypes to somatic and dendritic synapses in the central amygdala. Journal of Neurophysiology, 86 (2), 717-723.
Calcium-activated potassium currents in mammalian neurons
Sah, P and Davies, P (2000). Calcium-activated potassium currents in mammalian neurons. Clinical And Experimental Pharmacology And Physiology, 27 (9), 657-663. doi: 10.1046/j.1440-1681.2000.03317.x
Inhibition of transmitter release and long-term depression in the avian hippocampus
Margrie, TW, Rostas, JAP and Sah, P (2000). Inhibition of transmitter release and long-term depression in the avian hippocampus. Neuroscience Letters, 284 (1-2), 17-20. doi: 10.1016/S0304-3940(00)00992-7
Excitatory synaptic inputs to pyramidal neurons of the lateral amygdala
Mahanty, NK and Sah, P (1999). Excitatory synaptic inputs to pyramidal neurons of the lateral amygdala. European Journal of Neuroscience, 11 (4), 1217-1222. doi: 10.1046/j.1460-9568.1999.00528.x
Delaney, AJ and Sah, P (1999). GABA receptors inhibited by benzodiazepines mediate fast inhibitory transmission in the central amygdala. Journal of Neuroscience, 19 (22), 9698-9704.
Molecular studies of synaptic neurotran fission: Development of transgenic animal models
Morgan, B, Callister, R, Handford, C, Walker, S, Sah, P and Schofield, P (1999). Molecular studies of synaptic neurotran fission: Development of transgenic animal models. Journal of Neurochemistry, 73, S88-S88.
Sah, P and Clements, JD (1999). Photolytic manipulation of [Ca2+](i) reveals slow kinetics of potassium channels underlying the afterhyperpolarization in hipppocampal pyramidal neurons. Journal of Neuroscience, 19 (10), 3657-3664.
Use of murine mutants to study glycine receptor function
Callister, RJ, Schofield, PR and Sah, P (1999). Use of murine mutants to study glycine receptor function. Clinical And Experimental Pharmacology And Physiology, 26 (11), 929-931. doi: 10.1046/j.1440-1681.1999.03148.x
Are there functional P2X receptors on cell bodies in intact dorsal root ganglia of rats?
Stebbing, MJ, McLachlan, EM and Sah, P (1998). Are there functional P2X receptors on cell bodies in intact dorsal root ganglia of rats?. Neuroscience, 86 (4), 1235-1244. doi: 10.1016/S0306-4522(98)00127-4
Calcium-permeable AMPA receptors mediate long-term potentiation in interneurons in the amygdala
Mahanty, Nishith K. and Sah, Pankaj (1998). Calcium-permeable AMPA receptors mediate long-term potentiation in interneurons in the amygdala. Nature, 394 (6694), 683-687. doi: 10.1038/29312
Long-term potentiation of synaptic transmission in the avian hippocampus
Margrie, TW, Rostas, JAP and Sah, P (1998). Long-term potentiation of synaptic transmission in the avian hippocampus. Journal of Neuroscience, 18 (4), 1207-1216.
Neuroplasticity and psychiatry
Gynther, BD, Calford, MB and Sah, P (1998). Neuroplasticity and psychiatry. Australian And New Zealand Journal of Psychiatry, 32 (1), 119-128. doi: 10.3109/00048679809062718
Presynaptic long-term depression at a central glutamatergic synapse: a role for CaMKII
Margrie, TW, Rostas, JAP and Sah, P (1998). Presynaptic long-term depression at a central glutamatergic synapse: a role for CaMKII. Nature Neuroscience, 1 (5), 378-383.
Callister, RJ, Keast, JR and Sah, P (1997). Ca2+-activated K+ channels in rat otic ganglion cells: Role of Ca2+ entry via Ca2+ channels and nicotinic receptors. Journal of Physiology-london, 500 (3), 571-582. doi: 10.1113/jphysiol.1997.sp022043
NMDA receptor independent long-term potentiation in the avian hippocampus
Margrie, TW, Rostas, JAP and Sah, P (1997). NMDA receptor independent long-term potentiation in the avian hippocampus. Journal of Neurochemistry, 69, S66-S66.
The removal of acetylcholine by diffusion at nicotinic synapses in the rat otic ganglion
Callister, Robert J. and Sah, Pankaj (1997). The removal of acetylcholine by diffusion at nicotinic synapses in the rat otic ganglion. Journal of Physiology, 505 (1), 165-175. doi: 10.1111/j.1469-7793.1997.165bc.x
Sah, P and Bekkers, JM (1996). Apical dendritic location of slow afterhyperpolarization current in hippocampal pyramidal neurons: Implications for the integration of long-term potentiation. Journal of Neuroscience, 16 (15), 4537-4542.
Ca2+-activated K+ currents in neurones: Types, physiological roles and modulation
Sah, P (1996). Ca2+-activated K+ currents in neurones: Types, physiological roles and modulation. Trends in Neurosciences, 19 (4), 150-154. doi: 10.1016/S0166-2236(96)80026-9
Properties of Channels Mediating the Apamin-Insensitive Afterhyperpolarization in Vagal Motoneurons
Sah, P (1995). Properties of Channels Mediating the Apamin-Insensitive Afterhyperpolarization in Vagal Motoneurons. Journal of Neurophysiology, 74 (4), 1772-1776.
Sah, P and Isaacson, JS (1995). Channels Underlying the Slow Afterhyperpolarization in Hippocampal Pyramidal Neurons - Neurotransmitters Modulate the Open Probability. Neuron, 15 (2), 435-441. doi: 10.1016/0896-6273(95)90047-0
Sah, P and McLachlan, EM (1995). Membrane-Properties and Synaptic Potentials in Rat Sympathetic Preganglionic Neurons Studied in Horizontal Spinal-Cord Slices In-Vitro. Journal of the Autonomic Nervous System, 53 (1), 1-15. doi: 10.1016/0165-1838(94)00161-C
Sah, P (1995). Different Calcium Channels Are Coupled to Potassium Channels with Distinct Physiological Roles in Vagal Neurons. Proceedings of the Royal Society B-Biological Sciences, 260 (1357), 105-111. doi: 10.1098/rspb.1995.0066
Sah, P, Dulhunty, A, Junankar, P and Stanhope, C (1994). Subcellular-Distribution of Ryanodine Receptor-Like and Calcium Atpase-Like Immunoreactivity in Brain-Stem and Cerebellar Neurons of Rat and Guinea-Pig. Neuroscience Letters, 166 (2), 143-148. doi: 10.1016/0304-3940(94)90471-5
Sah, P, Francis, K, McLachlan, EM and Junankar, P (1993). Distribution of Ryanodine Receptor-Like Immunoreactivity in Mammalian Central-Nervous-System Is Consistent with its Role in Calcium-Induced Calcium Release. Neuroscience, 54 (1), 157-165. doi: 10.1016/0306-4522(93)90391-R
Jobling, P, McLachlan, EM and Sah, P (1993). Calcium Induced Calcium Release Is Involved in the Afterhyperpolarization in One Class of Guinea-Pig Sympathetic Neuron. Journal of the Autonomic Nervous System, 42 (3), 251-257. doi: 10.1016/0165-1838(93)90370-A
Sah, P and McLachlan, EM (1993). Differences in Electrophysiological Properties Between Neurons of the Dorsal Motor Nucleus of the Vagus in Rat and Guinea-Pig. Journal of the Autonomic Nervous System, 42 (2), 89-98. doi: 10.1016/0165-1838(93)90041-R
Sah, P (1992). Role of Calcium Influx and Buffering in the Kinetics of a Ca-2+-Activated K+ Current in Rat Vagal Motoneurons. Journal of Neurophysiology, 68 (6), 2237-2247.
Sah, Paj and McLachlan, EM (1992). Potassium Currents Contributing to Action-Potential Repolarization and the Afterhyperpolarization in Rat Vagal Motoneurons. Journal of Neurophysiology, 68 (5), 1834-1841.
A Slow Voltage-Activated Potassium Current in Rat Vagal Neurons
Sah, P and McLachlan, EM (1992). A Slow Voltage-Activated Potassium Current in Rat Vagal Neurons. Proceedings of the Royal Society B-Biological Sciences, 249 (1324), 71-76. doi: 10.1098/rspb.1992.0085
Sah, P and McLachlan, EM (1991). Ca2+-Activated K+-Currents Underlying the Afterhyperpolarization in Guinea-Pig Vagal Neurons - a Role for Ca2+-Activated Ca2+ Release. Neuron, 7 (2), 257-264. doi: 10.1016/0896-6273(91)90264-Z
Mechanisms Underlying Potentiation of Synaptic Transmission in Rat Anterior Cingulate Cortex Invitro
Sah, P and Nicoll, RA (1991). Mechanisms Underlying Potentiation of Synaptic Transmission in Rat Anterior Cingulate Cortex Invitro. Journal of Physiology-London, 433 (1), 615-630. doi: 10.1113/jphysiol.1991.sp018446
Properties of Excitatory Postsynaptic Currents Recorded Invitro From Rat Hippocampal Interneurons
Sah, P, Hestrin, S and Nicoll, RA (1990). Properties of Excitatory Postsynaptic Currents Recorded Invitro From Rat Hippocampal Interneurons. Journal of Physiology-London, 430, 605-616. doi: 10.1113/jphysiol.1990.sp018310
Properties of excitatory postsynaptic currents recorded in vitro from rat hippocampal interneurones
Sah, P, Hestrin, S and Nicoll, R A (1990). Properties of excitatory postsynaptic currents recorded in vitro from rat hippocampal interneurones. The Journal of Physiology, 430, 605-16. doi: 10.1113/jphysiol.1990.sp018310
Hestrin, S, Sah, P and Nicoll, RA (1990). Mechanisms Generating the Time Course of Dual Component Excitatory Synaptic Currents Recorded in Hippocampal Slices. Neuron, 5 (3), 247-253. doi: 10.1016/0896-6273(90)90162-9
Excitatory Synaptic Currents in Purkinje-Cells
Perkel, DJ, Hestrin, S, Sah, P and Nicoll, RA (1990). Excitatory Synaptic Currents in Purkinje-Cells. Proceedings of the Royal Society B-Biological Sciences, 241 (1301), 116-121. doi: 10.1098/rspb.1990.0074
A Voltage-Dependent Persistent Sodium Current in Mammalian Hippocampal-Neurons
French, CR, Sah, P, Buckett, KJ and Gage, PW (1990). A Voltage-Dependent Persistent Sodium Current in Mammalian Hippocampal-Neurons. Journal of General Physiology, 95 (6), 1139-1157. doi: 10.1085/jgp.95.6.1139
Hestrin, S, Nicoll, RA, Perkel, DJ and Sah, P (1990). Analysis of Excitatory Synaptic Action in Pyramidal Cells Using Whole-Cell Recording From Rat Hippocampal Slices. Journal of Physiology-London, 422, 203-225.
Physiological-Properties of Excitatory Synaptic Transmission in the Central-Nervous-System
Hestrin, S, Perkel, DJ, Sah, P, Manabe, T, Renner, P and Nicoll, RA (1990). Physiological-Properties of Excitatory Synaptic Transmission in the Central-Nervous-System. Cold Spring Harbor Symposia On Quantitative Biology, 55, 87-93.
Tonic Activation of Nmda Receptors by Ambient Glutamate Enhances Excitability of Neurons
Sah, P, Hestrin, S and Nicoll, RA (1989). Tonic Activation of Nmda Receptors by Ambient Glutamate Enhances Excitability of Neurons. Science, 246 (4931), 815-818. doi: 10.1126/science.2573153
Sah, P, Gibb, AJ and Gage, PW (1988). Potassium Current Activated by Depolarization of Dissociated Neurons From Adult Guinea-Pig Hippocampus. Journal of General Physiology, 92 (2), 263-278. doi: 10.1085/jgp.92.2.263
The Sodium Current Underlying Action-Potentials in Guinea-Pig Hippocampal Ca1 Neurons
Sah, P, Gibb, AJ and Gage, PW (1988). The Sodium Current Underlying Action-Potentials in Guinea-Pig Hippocampal Ca1 Neurons. Journal of General Physiology, 91 (3), 373-398. doi: 10.1085/jgp.91.3.373
Effects of Noradrenaline On Some Potassium Currents in Ca1 Neurons in Rat Hippocampal Slices
Sah, P, French, CR and Gage, PW (1985). Effects of Noradrenaline On Some Potassium Currents in Ca1 Neurons in Rat Hippocampal Slices. Neuroscience Letters, 60 (3), 295-300. doi: 10.1016/0304-3940(85)90593-2
Postsynaptic Effects of Some Central Stimulants At the Neuromuscular-Junction
Gage, PW and Sah, P (1982). Postsynaptic Effects of Some Central Stimulants At the Neuromuscular-Junction. British Journal of Pharmacology, 75 (3), 493-502. doi: 10.1111/j.1476-5381.1982.tb09166.x
Conference Publication
Anatomy and physiology of the central extended amygdala
Sun, Y., Turpin, F., Xu, L. and Sah, P. (2015). Anatomy and physiology of the central extended amygdala. 25th Biennial Meeting of the International Society for Neurochemistry Jointly with the 13th Meeting of the Asian Pacific Society for Neurochemistry in Conjunction with the 35th Meeting of the Australasian-Neuroscience-Society, Cairns, QLD Australia, 23-27 August 2015. Chichester, West Sussex United Kingdom: Wiley-Blackwell. doi: 10.1111/jnc.13189
Cortical gating of sensory responses in the amygdala
Windels, F., Yan, S., Stratton, P., Crane, J. and Sah, P. (2015). Cortical gating of sensory responses in the amygdala. 25th Biennial Meeting of the International-Society-for-Neurochemistry Jointly with the 13th Meeting of the Asian-Pacific-Society-for-Neurochemistry in Conjunction with the 35th Meeting of the Australasian-Neuroscience-Society, Cairns, QLD Australia, 23-27 august 2015. Chichester, West Sussex, United Kingdom: Wiley-Blackwell Publishing. doi: 10.1111/jnc.13188
Intrinsic circuitry of the lateral central amygdala
Hunt, S. and Sah, P. (2015). Intrinsic circuitry of the lateral central amygdala. 25th Biennial Meeting of the International Society for Neurochemistry Jointly with the 13th Meeting of the Asian Pacific Society for Neurochemistry in Conjunction with the 35th Meeting of the Australasian Neuroscience Society, Cairns, QLD Australia, 23-27 August 2015. Chichester, West Sussex, United Kingdom: Wiley-Blackwell Publishing. doi: 10.1111/jnc.13188
Reverberating cell assemblies in the amygdala
Perumal, M. B., Sullivan, R. and Sah, P. (2015). Reverberating cell assemblies in the amygdala. 25th Biennial Meeting of the International-Society-for-Neurochemistry Jointly with the 13th Meeting of the Asian-Pacific-Society-for-Neurochemistry in Conjunction with the 35th Meeting of the Australasian-Neuroscience-Society, Cairns, Australia, Aug 23-27, 2015. Chichester, United Kingdom: Wiley-Blackwell Publishing. doi: 10.1111/jnc.13189
Auditory stimulation modulates amygdala network dynamics
Windels, François, Stratton, Peter and Sah, Pankaj (2014). Auditory stimulation modulates amygdala network dynamics. The Twenty Third Annual Computational Neuroscience Meeting: CNS*2014, Québec City, Canada, 26-31 July, 2014. London, United Kingdom: BioMed Central. doi: 10.1186/1471-2202-15-s1-p52
Excitatory and inhibitory circuits in the basolateral amygdala
Sah, Pankaj (2012). Excitatory and inhibitory circuits in the basolateral amygdala. 19th Biennial Meeting of the International Society for Developmental Neuroscience (ISDN), Mumbai India, 11-14 January 2012. Oxford, United Kingdom: Pergamon. doi: 10.1016/j.ijdevneu.2012.10.085
Kidney chloride regulation in the mouse CNS
Sullivan, R. K. P., Spampanato, J. and Sah, P. (2011). Kidney chloride regulation in the mouse CNS. Australian Neuroscience Society (ANS) 31st Annual Conference, Aukland New Zealand, 30 January - 3 February 2011.
NMDA receptors in the amygdala: a heterogeneity of form and function
Sah, P (2010). NMDA receptors in the amygdala: a heterogeneity of form and function. Unknown, Unknown, Unknown. TOKYO: SPRINGER TOKYO.
Recent advances in the role of the amygdala in memory storage, fear and anxiety
Sah, P (2010). Recent advances in the role of the amygdala in memory storage, fear and anxiety. x, x, x. TOKYO: SPRINGER TOKYO.
Dendritic Branching and Excitatory Connectivity: Regulation by Seizure-Related Gene 6
Gunnersen, JM, Kim, MH, Fuller, SJ, De, SM, Barwood, JM, Hammond, VE, Britto, JM, Mateos, JM, Sonderegger, P, Faber, ES, Sah, P and Tan, SS (2009). Dendritic Branching and Excitatory Connectivity: Regulation by Seizure-Related Gene 6. 22nd Biennial Meeting of the International-Society-of-Neurochemistry/Asian-Pacific-Society-for-Neurochemistry, Busan South Korea, Aug 23-29, 2009. MALDEN: WILEY-BLACKWELL PUBLISHING, INC.
Fear conditioning and long-term potentiation in the amygdala: What really is the connection?
Sah, P., Westbrook, R. F. and Lüthi, A. (2008). Fear conditioning and long-term potentiation in the amygdala: What really is the connection?. Blackwell Publishing Inc.. doi: 10.1196/annals.1417.020
Interneurons in the basolateral amygdala: A diversity of form and function
Woodruff, A., Polepalli, J., Yanagawa, Y. and Sah, P. (2008). Interneurons in the basolateral amygdala: A diversity of form and function. Eighth Biennial Meeting of the Asia-Pacific Society for Neurochemistry, Shanghai, Peoples Republic of China, 23-26 June 2008. Oxford, England, U. K.: Wiley-Blackwell Publishing Ltd.. doi: 10.1111/j.1471-4159.2008.05367.x
Distribution and function of GABA(A) receptor subunits in the amygdala
Esmaeili, Abolghasem, Lynch, Joe and Sah, Pankaj (2006). Distribution and function of GABA(A) receptor subunits in the amygdala. The 29th Annual Meeting of the Japan Neuroscience Society - (Neuroscience2006), Kyoto, Japan, 19-21 July 2006. Ireland, U.K.: Elsevier Ireland. doi: 10.1016/j.neures.2006.04.004
Woodruff, A.R. and Sah, P. (2004). A chemically and electrically connected network of interneurons regulates principal neuron activity in the basolateral amygdala. Australian Neuroscience Society 24th Annual Meeting, Melbourne Convention Centre, 27-30 January. Melbourne: Australian Neuroscience Society.
Faber, E.S.L. and Sah, P. (2004). Calcium and voltage dependent potassium currents contribute to spike frequency adaptations in lateral amygdala neurons. Australian Neuroscience Society 24th Annual Meeting, Melbourne Convention Centre, 27-30 January. Melbourne: Australian Neuroscience Society.
Stimulation of Girk channel activity by P75 neurotrophin receptor (p 75NTR) promotes neuronal death
Coulson, E., Sah, P., May, L. M., Morley, S. N. and Bartlett, P. F. (2004). Stimulation of Girk channel activity by P75 neurotrophin receptor (p 75NTR) promotes neuronal death. Society for Neuroscience 34th Annual Meeting, San Diego, 23-27 October, 2004. Washington DC: Society for Neuroscience.
Research Areas
- Electrophysiology and molecular techniques
- Neural circuitry underpinning learning and memory formation
- The amygdala and fear conditioning
- Deep brain stimulation (DBS)